Is There a Protective Effect of Sequential Grafting in Coronary Bypass Surgery Using Bilateral Internal Thoracic Artery?
Abstract
Objectives: It is unclear whether the additional bypass techniques to supplement bilateral internal artery grafting (BITA) influence the patient outcome in coronary surgery. We analyzed the impact of sequential ITA grafts on late survival after BITA used on the left side.
Methods: From 1989 to 2014, 1644 patients underwent BITA surgery without any additional arterial graft. The revascularization of the left side was optimized with a sequential ITA graft in 824 patients. The revascularization of the right side was performed with an associated vein graft in 599 patients. Propensity score was calculated by logistic regression model and patients were matched 1 to 1 leading to two groups of 334 matched patients. The primary end point was overall mortality from any cause.
Results: The population was not homogenous: Greater the arterial revascularization, lower the risk profile. The 30-day mortality was 1.2% without influence of the surgical technique performed. The mean postoperative follow-up was 12.4 ± 6.7 years and 95% complete. Late mortality was significantly influenced by age, heart failure, 3-vessel disease, LV ejection fraction, number of arterial anastomoses and sequential ITA. The significant difference in patients' survival observed at 20-years in favour of sequential ITA in unmatched groups was confirmed in matched groups. In multivariable Cox model analysis, the use of sequential ITA remained predictor of long-term survival in matched groups.
Conclusions: These results confirm that higher the number of ITA anastomoses, better the long-term survival. It is a strong support of the extensive use of arterial grafting with multiple ITA bypass.
Keywords
Coronary disease, Arterial revascularization, Internal thoracic artery, Sequential graft
Introduction
Long-term advantages of multiple arterial grafting in patients undergoing coronary artery bypass surgery (CABG) has been controversial for decades despite mounting of evidence supporting the use of this technique for myocardial revascularization [1-3]. Nowadays, there is a consensus to use bilateral internal thoracic artery (BITA) to bypass the left coronary network [4] and ESC/EACTS 2018 guidelines on myocardial revascularization recommend the use of BITA grafting in patients who do not have a high risk of sternal wound infection [5]. However, the benefits of total arterial revascularization and the associated techniques to supplement BITA have remained controversial [6,7]. In this retrospective study based on our 25-year experience in arterial grafting, we have analyzed the long-term survival after in situ BITA grafting without additional arterial graft to test the hypothetic impact of the sequential ITAs on the left side, used to perform an extensive arterial myocardial revascularization.
Materials and Methods
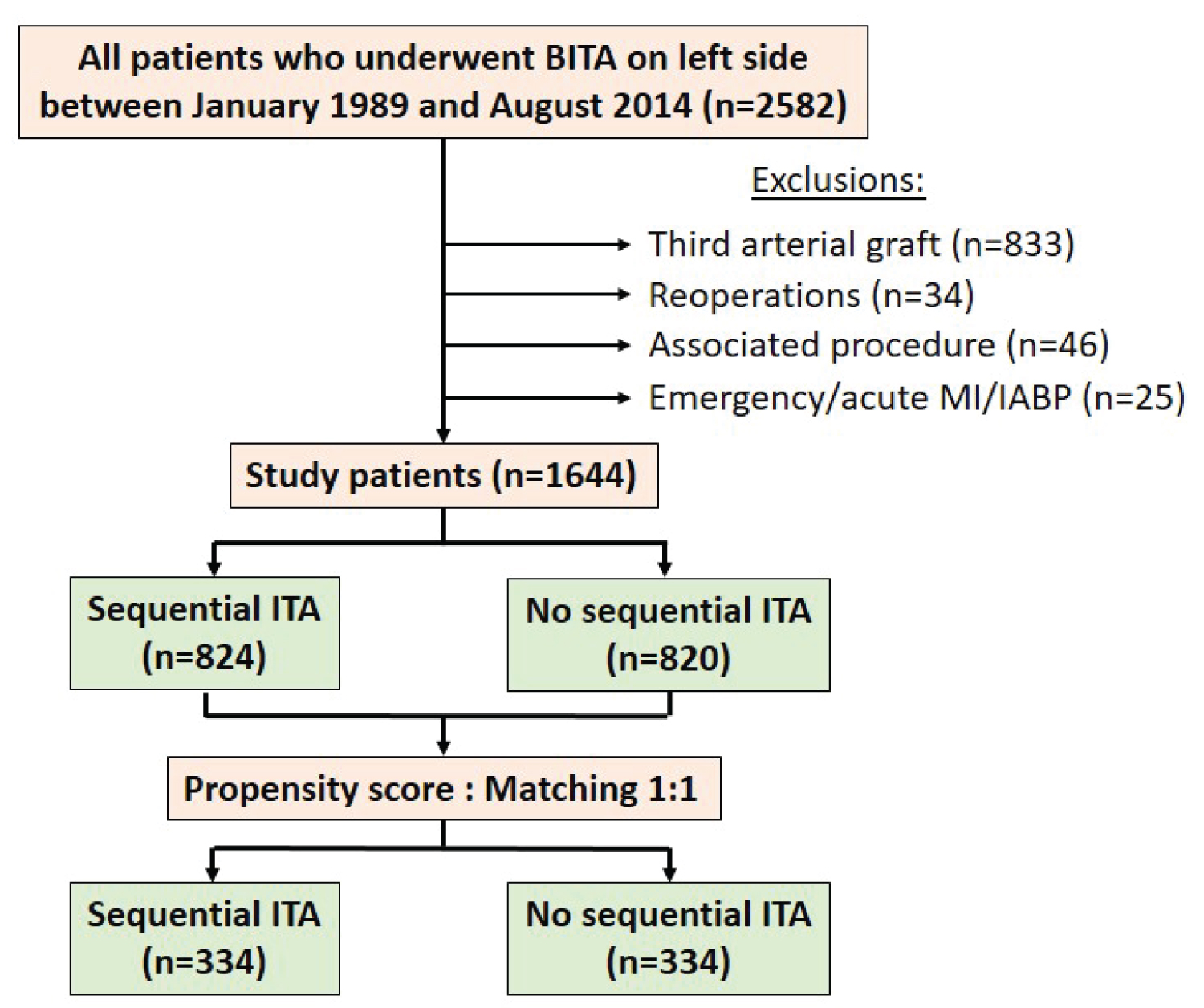
All patients who underwent isolated CABG procedure using BITA in our department performed by the same surgeon from January 1989 to August 2014 were selected in the study. The inclusion criteria were BITA on the left side regardless if sequential ITA was used, associated or not with a vein graft on the right side. Exclusion criteria were emergency, reoperation, associated procedure, unstable situation and additional arterial graft. We retrospectively analyzed prospectively collected data from the surgical registry of the department approved by the local ethical committee and receiving individual patient consent.
Surgical technique
Our surgical techniques in CABG were previously reported in studies focused on early postoperative outcome [8]. As arterial grafting is technically more demanding, particularly in patients with left ventricular (LV) dysfunction or obesity, the main concern was no increase in mortality or morbidity. Diabetes status or severe dyslipidemia were never a limitation. CABG was done on-pump with ante grade and retrograde crystalloid cardioplegia. Patients received both left and right internal thoracic artery (LITA and RITA) to the most important coronary arteries on the left side: RITA crossing in front the aorta to the left anterior descending artery (LAD) and LITA to the circumflex artery (CX) system; sequential ITA grafts were performed according to the coronary lesions and the technical possibilities: Mainly sequential LITA to diagonal and marginal branches. A supplemental vein graft was used to bypass the right coronary artery (RCA) system, as needed. All ITA grafts were used as in situ grafts preferentially with thin pedicle; skeletonization was not systematic and done to increase the length of the graft when necessary; composite ITA grafts were exceptionally used. Complete myocardial revascularization was defined as bypass of all significant lesions defined as more than 70% stenosis. All patients received aspirin antiplatelet therapy postoperatively. Postoperative statin and beta-blockers became common practice over the years.
Definitions and end point
Early mortality was defined as any death within 30 days of CABG. Late death was defined as death occurring after 30 days from surgery. All causes mortality was used to assess long-term outcome. The last survival status of the patients was obtained in 2019 from the National institute of statistics and economic studies (INSEE) and a genealogy agency in case of lack of information; the common closing date for follow-up was December 1, 2019. The primary end point was overall mortality from any cause and was analyzed according to the potential risk factors and the surgical configuration.
Statistical analysis
Descriptive statistics for categorical variables are reported as number and percentage; continuous variables are reported as mean ± standard deviation. Continuous variables were compared using Student' t-test and ANOVA; categorical variables were compared using χ2 or Fisher's exact test. Overall survival was estimated using the Kaplan-Meier method and reported as percentage (95% confidence interval). The stratified log rank test was applied to compare the equality of the survival curves; actuarial survival was reported on tables and curves. Univariable analyses of predictors of all-cause death were done with binary logistic regression. Propensity-score matching was performed to correct for the bias associated with the use of sequential ITA graft. A propensity score for each patient was calculated by logistic regression model with sequential ITA graft as the dependent variable and age, gender, NYHA status, left main stenosis, diabetes, left ventricular ejection fraction (LVEF), complete revascularization, an associated vein on the RCA system, the operative times (clamp time and cardiopulmonary time) and the year of surgery as independent variables. Patients were matched 1 to 1 on their propensity score using the greedy matching method without replacement and a fixed caliper width of 0.005. All potentially important variables were first individually tested by a log-rank test of the corresponding Kaplan-Meir survival curves and then they were included in a multivariable Cox regression analysis to identify independent predictors of survival in matched groups.
Statistical analyses were based on variables documented and complete in all patients. A 2-tailed P value < 0.05 was always considered to indicate statistical significance. All statistical analyses were performed using IBM-SPSS Statistics software version 25.0 (IBM-SPSS Inv, Armonk, NY).
Results
Finally, 1644 patients were included in the study: 1045 patients had isolated BITA, 599 patients had BITA with an associated vein graft on the right side and a sequential ITA graft was performed in 824 patients (Figure 1).
In unmatched population, a sequential ITA graft was used significantly more in males, in patients with diabetes and in patients with a preserved left ventricular function. In sequential ITA group, number of distal anastomoses, number of arterial anastomoses, operative times were significantly higher, and a vein graft was less associated without difference in completeness of revascularization between both groups (Table 1). In this series, the early mortality was 1.2% and it was not significantly influenced by the surgical technique used, like postoperative complications rate (Table 2). The mean postoperative follow-up was 12.4 ± 6.7 years, and 95% complete: 805 late deaths occurred (mean delay 10.6 ± 6.2-years), 734 patients were alive (mean follow-up 14.4 ± 6.6-years) and 85 patients were lost of follow-up (37 patients during the first postoperative year, and 48 patients after 5.8 ± 2.6-years).
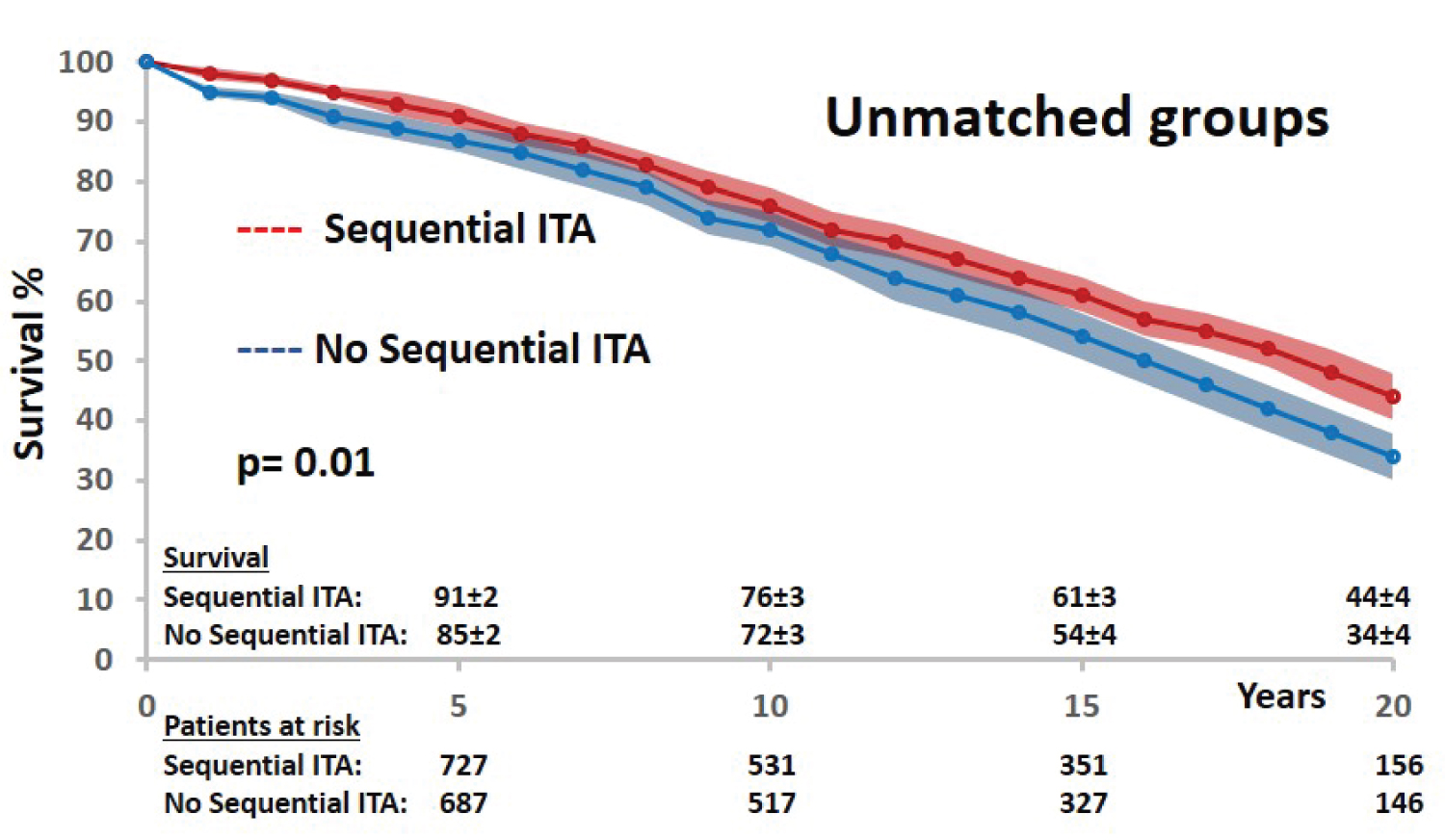
The long-term survival after BITA grafting was significantly influenced by the technical configuration with a 10% difference at 20-years in favor of sequential ITA use (Figure 2). Several preoperative and intraoperative variables were identified as significant predictors of all causes mortality by univariable analysis: Age, heart failure, 3-vessel disease status, LV ejection fraction, number of arterial anastomoses, and sequential ITA graft. Gender, diabetes status, left main lesion, completeness of revascularization and operative times were not significant prognosis factors of mortality (Table 3).
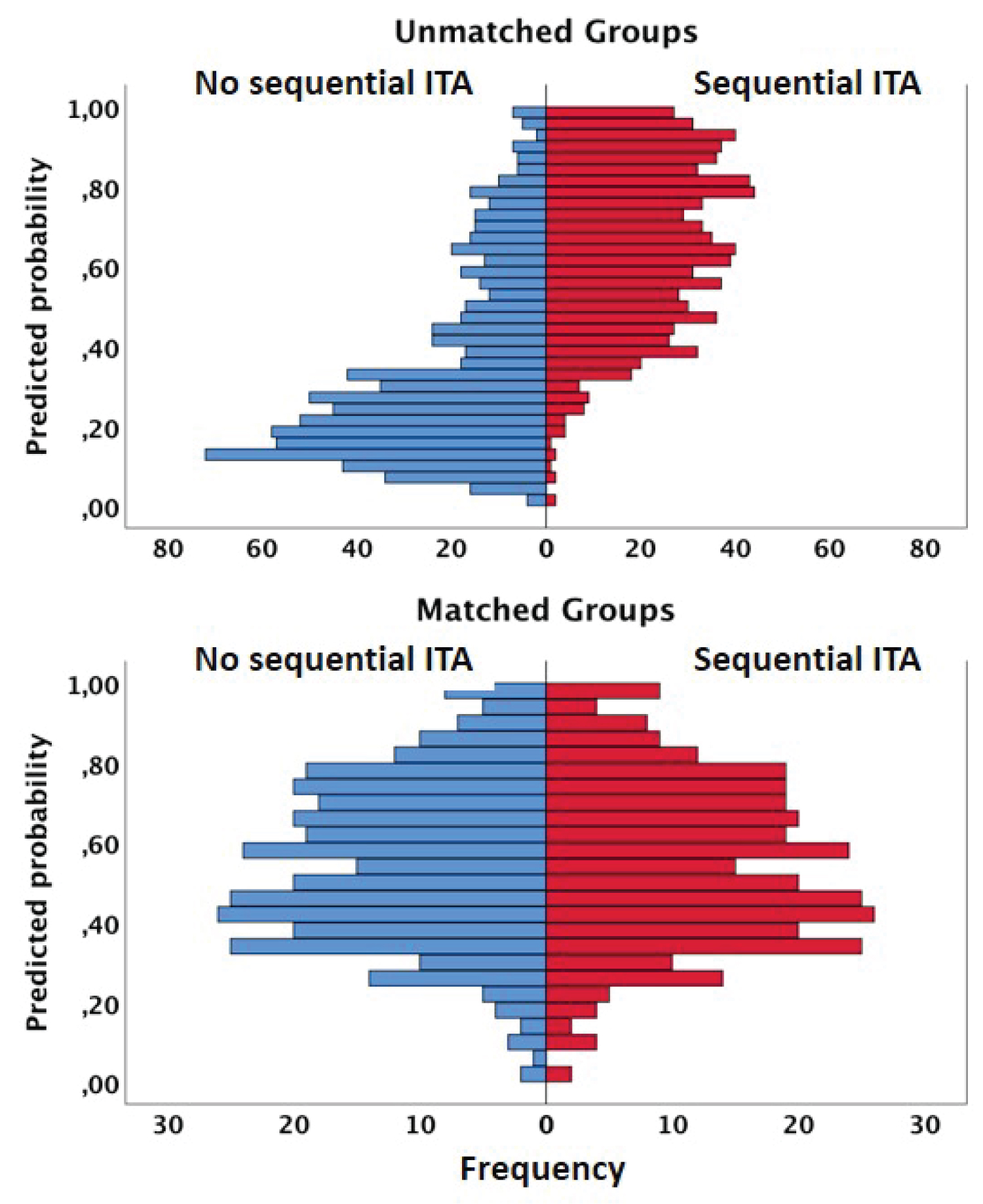
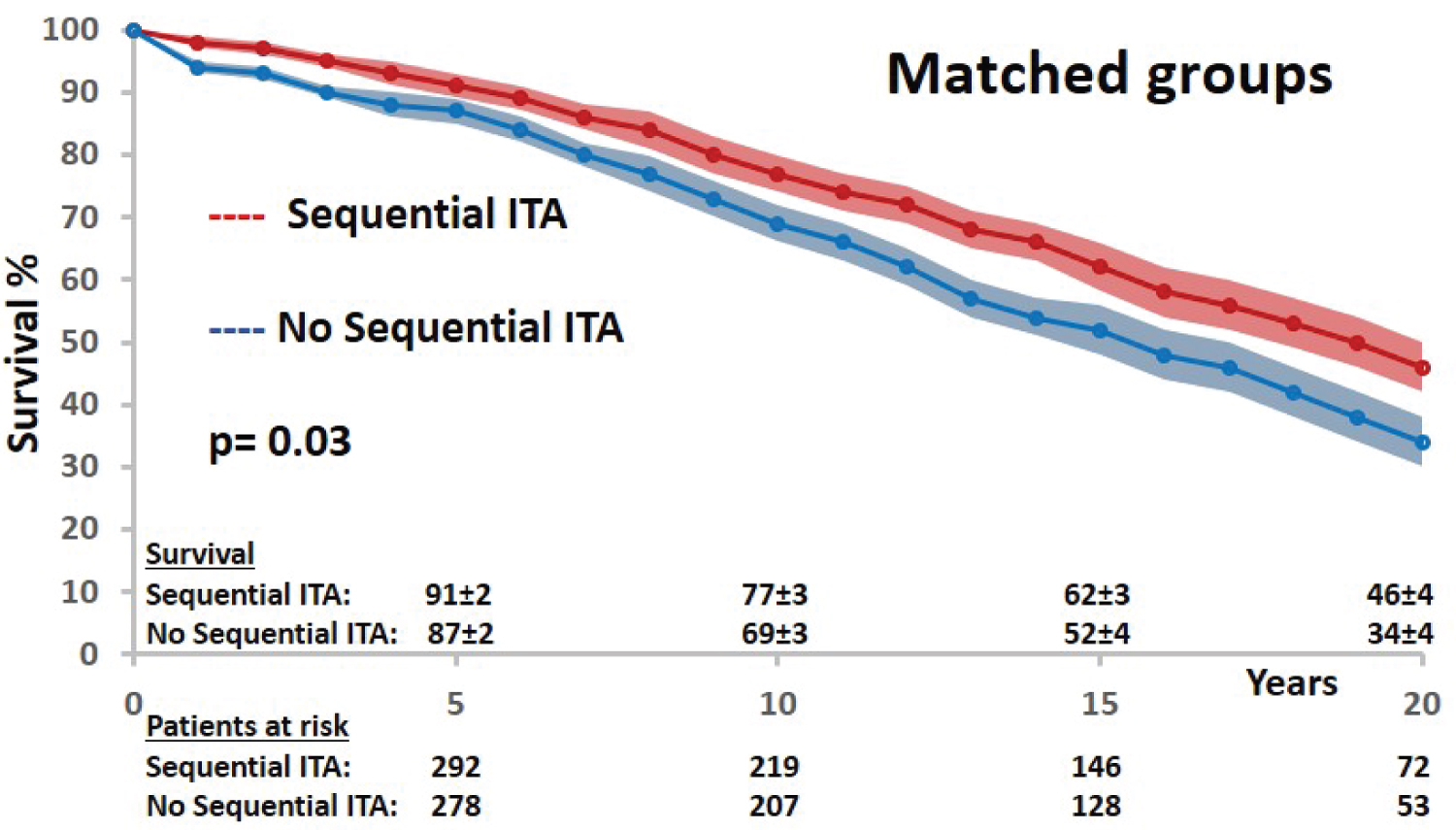
In matched groups, there was no more difference in preoperative characteristics between both groups, confirming an optimal matching model according to propensity score distributions (Figure 3) and standardized median differences (Table 1). Obviously, the two groups were not matched according to the number of distal and arterial anastomoses. The early postoperative outcome was similar in both groups (Table 2). The late survival was significantly influenced by age, heart failure, diabetes status, 3-vessel disease status and LV ejection fraction (Table 4). Regarding the technical configuration, the late survival was significantly different among matched groups in favor of sequential grafting (Mantel-Cox chi-square: 5.069, p = 0.03; Figure 4). In multivariable analysis with Cox regression model (Chi-square: 204,030; df: 11; p = 0.0001), age, heart failure and LV ejection remained independent risk factors of survival; regarding operative and technical criteria, only the use of sequential ITA graft was a significant independent prognosis factor of survival; complete revascularization and RCA bypass (vein graft or no graft) were not identified as independent prognosis factors (Table 5). According to these results, only the revascularization of the left coronary network by multiple ITA anastomoses with sequential technique had an impact on long-term survival, predominant over complete revascularization and independently of the RCA revascularization (vein graft or no graft).
Discussion
Multiple graft configurations have been described to use both ITA, including in situ and free grafts, and patency rates for both LITA and RITA have been shown to be equivalent for specific myocardial territories, and better than radial or saphenous vein grafts [7,9]. Because the additional risk has been sufficiently low and the potential benefit could be a life expectancy improvement, in 1989 we estimated that our patients have deserved a shift in our practice of coronary revascularization and we decided to perform BITA grafting and multiple arterial revascularization as often as possible. Our strategy was to tailor the operation to the patient according to the coronary network and the estimated operative risk to avoid an increase of early mortality. Our preference has been pedicled ITA grafts to bypass the left coronary artery system with wide use of sequential ITA graft, and an additional vein or arterial graft to the right side. Our previous reports focused on short-term and mid-term results, demonstrated that this strategy was safe even in diabetes patients with a low rate of sternal wound infection [8]. The primary endpoint of this retrospective observational study conducted in one institution and based on a single surgeon experience, was focused on the overall mortality with a mean postoperative follow-up of 12-years. The aim of the study was focused on the impact of sequential ITA and patients who underwent an additional arterial graft to BITA were excluded to avoid an additional bias. In unmatched and matched groups, univariate and multivariate analyses have confirmed the traditional prognosis factors of mortality after CABG [10,11]. Regarding the arterial grafting configuration and the technical strategy, the bypassing of multiple target coronary vessels of the left territory with sequential ITA grafts was a significant independent predictor of late survival, predominant over complete revascularization and the revascularization of the RCA territory, with a 12% difference at 20-years between the matched groups, in favor of sequential ITA graft . However, the interpretation of the results remains tricky because there was no alternative to sequential ITA bypass, and it is difficult to know if the impact we have observed is related to an obvious additional revascularization or to its ITA origin. Interestingly, there was no difference in early mortality and morbidity after CABG according to the surgical technique performed, confirming a posteriori the efficiency of the strategy of revascularization we adopted and that judicious patient selection for a given grafting strategy is important for optimal outcomes.
In many studies focused on both ITA grafting, the impact of sequential ITA graft is not clear, the non-LAD target vessels bypassed by additional arterial graft are variable, in situ or free arterial graft are mixed and the number of distal arterial anastomoses are not mentioned; so finally the unicity of the surgical technique is not enough to determine a consistent impact. In our experience, sequential ITA grafts were used to increase the number of distal arterial anastomoses and to perform extensive arterial grafting on the left side. Benefits of sequential ITA are well established [11,12]. Kieser, et al. [13] have reported that a strategy of multiple ITA grafts may balance survival between complete and incomplete revascularization that is one of our result as well. Yanagawa, et al. [2] have reported a meta-analysis showing that total arterial revascularization may improve long-term survival even when compared with two arterial grafts, suggesting that higher the number of arterial anastomoses better the survival. The better patency of ITA grafts has been involved to explain such positive impact [12]. The hypothesis that arterial grafts has a strong protective effect against progression of native coronary artery disease in previously grafted vessels has been proposed and multiple arterial grafting may improve long-term survival by preventing progression of atherosclerosis in the native coronary vessels with a possible NO mediation [14]. More recently, Bakaeen, et al. [15] showed that in BITA grafting, bypassing multiple targets to maximize myocardium mass supplied by ITAs, improved long-term survival. According to our results and the preceding studies, we can speculate that an extended ITA revascularization of the left coronary network with sequential BITA may have a protective effect on the long-term outcome related to preventing the progression of the atherosclerosis of bypassed vessels and related to a bigger myocardium mass supplied by ITAs, despite the fact that patient who underwent sequential ITA had initially more severe coronary lesions. This hypothesis is consistent with our principal finding in the matched group analysis, that the impact of sequential ITA is predominant over complete revascularization and the RCA grafting options; patients without sequential ITA had additional lateral vessels either no sick and exposed to a progression of the disease or too sick to be bypassed and exposed to an incomplete revascularization, that was balanced in the propensity score matching. Pevni, et al. [16] have already shown that the revascularization of the RCA independently of the graft used had no impact on the late survival of patients who underwent BITA grafting on the left side, which is consistent with our results as well.
Despite its inherent limitations, our study has demonstrated that BITA grafting is not an end in itself: Higher the number of ITA anastomoses with the use of sequential ITA, greater the myocardial mass perfused by ITA grafts, better the long-term survival. RCA revascularization seems having no impact on survival after BITA grafting, without prejudging a possible impact on late cardiac events.
Limitations
The present study has several limitations, inherent to its design and objectives. This is a retrospective observational nonrandomized study based on a 25-year single center, single surgeon and single technical configuration operative experience. Only solid preoperative characteristic and documented for all patients were integrated in the risk factors analysis: For example Euroscore or STS score were missing before 2000, obesity status was not defined properly according to BMI, and they were not included. Nevertheless, the preoperative characteristic included are recognized as the main risk factors of CABG, defining well our CABG population and discriminant in comparisons between the multiple arterial grafting strategies performed in patients. The operative parameters were more exhaustive, more precise, allowing a robust analysis of the operative configuration and its impact. Only long-term survival and all-cause mortality were defined as primary end-point of the study. It was not the intent of the study to report on other major adverse events as myocardial infarction, repeat revascularization, cause of death, or on graft patency, and the collection of these information was not realistic in this retrospective study over 30-years. Propensity-score matching was performed to correct for the bias associated with the use of sequential ITA graft. The residual bias related to the absence of alternative to sequential ITA graft in our strategy, was probably mitigated by the balance in complete revascularization and RCA bypass in the two matched groups; however it makes the interpretation of the results questionable. Therefore, we expect confirmation from the ROMA (Randomized Comparison of the Clinical Outcome of Single versus Multiple Arterial Grafts) trial under way [17]. Nevertheless, we can distill our finding to practical recommendations. In patients who are selected for BITA grafting, use the ITAs to revascularize the greatest total myocardium with the added-value of sequential technique; the more myocardium supplied by ITAs, the better the survival.
Conclusions
According to the results of this study, sequential ITA graft is an independent predictor of late survival after BITA grafting on the left side, predominant over the completeness of the revascularization. These findings support the concept that extensive use of ITA grafts should be the cornerstone of modern coronary artery surgery. However they have to be confirmed in further studies.
Acknowledgement
We thank Isabelle Porta, Rosalie Tchapnou and Raphael Riou for their assistance in the collection of follow-up data.
Conflict of Interest
None declared.
Funding Statement
None declared.
Data Availability
The data that support the finding of this study are available from the corresponding author, upon reasonable request.
Ethics Approval and Consent to Participate
Ethics approval and consent to participate are mentioned in Methods.
References
- Lytle BW, Blackstone EH, Loop FD, et al. (1999) Two internal thoracic artery are better than one. J Thorac Cardiovasc Surg 117: 855-872.
- Yanagawa B, Verma S, Mazine A, et al. (2017) Impact of total arterial revascularization on long term survival: A systematic review and meta-analysis of 130,305 patients. Int J Cardiol 233: 29-36.
- Buttar SN, Yan TD, Taggart DP, et al. (2017) Long-term and short-term outcomes of using bilateral internal mammary grafting versus left internal mammary artery grafting: A meta-analysis. Heart 103: 1419-1426.
- Pevni D, Mohr R, Kramer A, et al. (2019) Are two internal thoracic grafts better than one? Eur J Cardiothorac Surg 56: 935-941.
- Sousa-Uva M, Neumann FJ, Ahlsson A, et al. (2019) 2018 ESC/EACTS guidelines on myocardial revascularization. Eur J Cardiothorac Surg 55: 4-90.
- Glineur D, D'hoore W, Price J, et al. (2012) Survival benefit of multiple arterial grafting in a 25-year single institutional experience: The importance of the third arterial graft. Eur J Cardiothorac Surg 42: 284-290.
- Shi WY, Tatoulis J, Newcomb AE, et al. (2016) Is a third arterial conduit necessary? Comparison of the radial artery and saphenous vein in patients receiving bilateral internal thoracic arteries for triple vessel coronary disease. Eur J Cardiothorac Surg 50: 53-60.
- Jegaden O, Eker A, Montagna P, et al. (1995) Risk and results of bypass grafting using bilateral internal mammary and right gastroepiploic arteries. Ann Thorac Surg 59: 955-960.
- Raja SG, Benedetto U, Hussain M, et al. (2014) Does grafting of the left anterior descending artery with the in situ right internal thoracic artery have an impact on late outcomes in the context of bilateral internal thoracic artery usage? J Thorac Cardiovasc Surg 148: 1275-1281.
- Yanagawa B, Verma S, Juni P, et al. (2017) A systematic review and meta-analysis of in situ versus composite bilateral internal thoracic artery grafting. J Thorac Cardiovasc Surg 153: 1108-1116.
- Parsa CJ, Shaw LK, Rankin S, et al. (2013) Twenty-five-year outcomes after multiple internal thoracic artery bypass. J Thorac Cardiovasc Surg 145: 970-975.
- Raza S, Blackstone EH, Bakaeen FG, et al. (2019) Long-term patency of indivudual segments of different internal thoracic artery graft configurations. Ann Thorac Surg 107: 740-746.
- Kieser TM, Curran HJ, Rose MS, et al. (2014) Arterial grafts balance survival between incomplete and complete revascularization: A series of 1000 consecutive coronary artery bypass graft patients with 98% arterial grafts. J Thorac Cardiovasc Surg 147: 75-84.
- Dimitrova KR, Hoffman DM, Geller CM, et al. (2012) Arterial grafts protect the native coronary vessels from atherosclerotic disease progression. Ann Thorac Surg 94: 475-481.
- Bakaeen FG, Ravichandren K, Blackstone EH, et al. (2020) Coronary artery target selection and survival after bilateral internal thoracic artery grafting. J Am CollCardiol 75: 258-268.
- Pevni D, Uretzky G, Yosef P, et al. (2005) Revascularization of the right coronary artery in bilateral internal thoracic artery grafting. Ann ThoracSurg 79: 564-569.
- Gaudino M, Alexander JH, Bakaeen FG, et al. (2017) The randomized comparison of the clinical outcome of Single versus multiple arterial grafts: The ROMA trial-rationale and study protocol. Eur J Cardiothoracic Surg 52: 1031-1040.
Corresponding Author
Olivier JEGADEN, Department of Cardiac Surgery, Mediclinic Airport Road Hospital, PO Box 48481, Abu Dhabi, UAE, Tel: +971554595891.
Copyright
© 2021 Alshamri A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.