Human Left Atrial Potassium Ion Channel Transcripts in Patients Requiring Cardiac Surgery vs. Structurally Normal Hearts: Potential Implications for the Mechanisms of Post-Operative Atrial Fibrillation
Abstract
Atrial Fibrillation (AF) is the most common arrhythmia following cardiac surgery, and contributes significantly to the post-operative morbidity. However, an understanding of the molecular changes to the left atrium in patients requiring cardiovascular surgery, which place them at greater risk for developing post-operative AF is not completely understood. We hypothesized that there would be significant differences in K+ channel transcript expression between patients with cardiovascular disease requiring surgery and those with a structurally normal heart. Left atrial tissue biopsies were obtained from 10 patients with a structurally normal heart and compared to previously obtain left atrial biopsies from patients having cardiac surgery. RT-PCR examined the expression of K+ channel transcripts between patients with and without cardiovascular disease. Specimens were stained with hematoxylin and eosin and picrosirius red to quantify the percentage of fatty infiltration and fibrosis respectively confirming that the left atrial tissue was devoid of cardiovascular disease. Fifty patients, 40 with cardiovascular disease requiring surgery, 10 of which developed AF, and 10 patients with a structurally normal heart were compared. Histology confirmed the absence of fibrosis in all structurally normal left atrial samples. The expression of Kir 2.3, 3.1, 3.4 and Kv 1.5 were all significantly greater within the structurally normal heart, with no specific differences between those with cardiovascular disease and either remained in normal sinus rhythm or who developed AF. This demonstrates K+ channel transcript differences between patients requiring cardiac surgery and those with a structurally normal heart.
Keywords
Atrial fibrillation, Ion channel, Fibrosis
Abbreviations
CVD: Cardiovascular Disease
Introduction
Atrial Fibrillation (AF) is the most common arrhythmia following cardiac surgery, and contributes significantly to the post-operative morbidity and mortality [1-4]. The left atrium maintains AF following surgery [1] and we have previously demonstrated that there are significant structural differences in the left atrium of patients who develop post-operative AF, particularly fibrosis [1,5,6]. In addition, our previous work demonstrated a difference in K+ channel transcripts between left and right atrial tissue in all patients with cardiovascular disease requiring surgery, without significant differences between those who remained in NSR and developed AF [1]. A gradient in K+ channel transcripts between the left vs. right atrium may serve to shorten the effective refractory period creating conditions favorable for the stabilization of rotors in all patients requiring surgery, implicating the greater importance of other proposed mechanisms (Inflammation, fibrosis, etc).
However, a comparison of the expression of K+ channel transcripts in patients without cardiovascular disease and with a structurally normal heart was not possible at that time of our previous publication [1]. This comparison is essential to confirm that when compared to the structurally normal heart, patients who developed AF did not have any significant changes in K+ channel expression.
Potassium ion channel transcripts can significantly alter the action potential, creating conditions favorable for both the initiation and maintenance of post-operative AF. Particularly changes in Kir 3.1 and 3.4 which govern IKACh can shorten the action potential duration, and refractory period allowing for faster repolarization [6,7]. In addition, changes to Kir 2.1 and 2.3 which govern IK1, determine the resting membrane potential [8]. These transcripts [6,7] and others [8] have been implicated in the development of AF in animals [7]. However, data comparing these transcripts between those who developed AF or remained in NSR following surgery to the structurally normal heart are scant.
The purpose of the current study was to quantify the expression of K+ channel transcripts from human left atrial tissue in the structurally normal heart compared to a cohort of patients previously enrolled for surgery [1]. We hypothesized that there would be significant differences in K+ channel transcript expression between all patients with cardiovascular disease and those with a structurally normal heart.
Methods
Following Institutional Review Board approval, a subset of patients who were previously reported [1] that required surgery were compared to a group of patients at the time of organ donation. A left atrial biopsy was obtained at the time of organ donation following review by the Finger Lakes Donor Network Organ Procurement Organization and family consent for the use of organs and tissue for research purposes. The left atrial appendage was selected as the point of biopsy due to its increased ease in surgical closure within patients requiring cardiac surgery, and its relative unimportance and common area for venting during cardiectomy. Briefly, following the initiation of cardiopulmonary bypass, and cardioplegic arrest, approximately a 0.5 × 0.5 cm biopsy was taken from the left atrial appendage, and the defect sutured using a 5-0 prolene. A similar sized biopsy was taken from the left atrial appendage at the time of organ donation, following cardiectomy. The tissue was immediately placed in 1 µl of RNA later (Thermo Fisher, Waltham) for 12 hours, and frozen at -80 ℃.
Clinical data
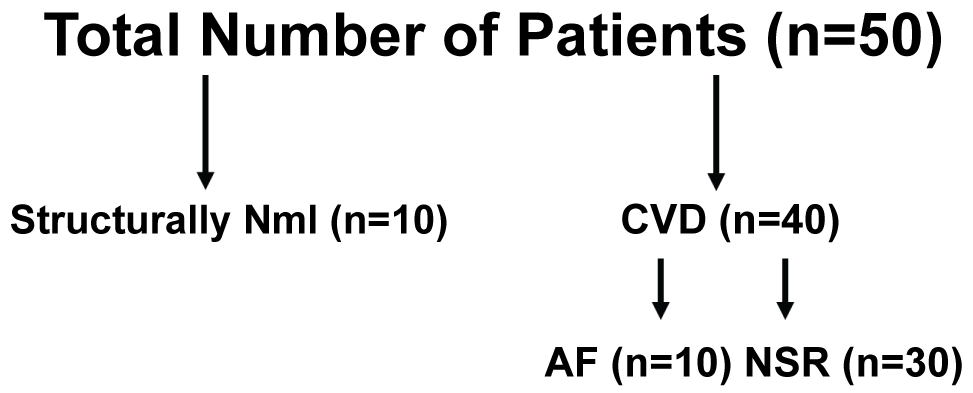
In all cases, pre-operative demographics were recorded on all patients. A pre-operative echocardiogram was used to measure the left ventricular ejection fraction. In addition, the presence or absence of diastolic dysfunction was also compared between groups. Left atrial biopsies were obtained from 50 patients, all without a known history of AF. Forty had significant cardiovascular disease requiring surgery, and 10 were from a structurally normal heart (Figure 1). From those patients requiring cardiac surgery, 10 developed post-operative AF. Samples were also divided into three groups: 1) Cardiovascular disease requiring surgery and developed post-operative AF; 2) Cardiovascular disease requiring surgery and remained in normal sinus rhythm and 3) Structurally normal hearts used for cardiac transplant.
K+ channel transcript PCR
An RN easy kit (Qiagen, Hilden) was used to extract the RNA from each sample. Following RNA extraction, samples were stored at -80 ℃ to prevent continued degradation until the time of use. Primers were designed to cross an intron, and the forward and reverse primers (Table 1) were reconstituted with RNA se free water. A SSIII RT kit (Invitrogen, Waltham) was used according to the manufacturer's specifications to reverse transcribe 50 ng of RNA. RT-PCR was performed using an Express SYBR Green ER q PCR Super Mix (Thermo Fisher, Waltham), in triplicate on a 48 well plate. Data was normalized to GAPDH using the ΔΔ cT (cycle to threshold) method [8]. The lowest Δ cT value was then used as a standard, and subtracted from each sample's Δ cT value to result in a ΔΔ cT value. The fold increase was then determined by using the corresponding equation.
Δ cT = (cT value for the gene of interest) - (cT value for GAPDH)
ΔΔ cT = (Gene of interest Δ cT) - (Lowest Δ cT value for that gene within the cohort)
Fold Increase = 2 -ΔΔ cT
Structural heterogeneities
To confirm, that the left atrial tissue was from a structurally normal heart, histology was preformed looking for structural heterogeneities. The left atrial biopsies were fixed in 10% formalin, imbedded, and sectioned at 6 µm. Slides were stained with hematoxylin and eosin and examined at 10X to measure the left atrial muscle cell diameter. Data was obtained from 100 cells per sample. In addition, the presence and percentage of fatty infiltration per sample were also determined. Fatty infiltration was defined as greater than 2 adipocytes surrounded by muscle. Last, slides were stained with picrosirius red to qualify and quantify the presence of fibrosis at 10X. Fibrosis was defined as interdigitating strands located between muscle bundles. Evidence of fibrosis between muscle bundles was classified as positive for the presence of interstitial fibrosis. All microscopy was performed by two blinded investigators using a Leica Microscope, and image files saved and analyzed.
Statistical analysis
Data is presented as a mean ± the standard deviation or as a frequency and percentage. All data was compared using one-way ANOVA. Categorical variables were compared using a Fisher's exact test. A p value of < 0.05 was considered significant. All authors agree with the manuscript as written.
Results
Patient demographics
From the biopsies obtained, the majority of patients requiring surgery had coronary artery disease (n = 16) and/or isolated valve disease (n = 7), and there were no significant differences in those who developed AF vs. remained in NSR in the type of disease or corresponding operation required. Pre-operative demographics between those who required surgery and those with a structurally normal heart are listed in Table 2. The average age of patients with a structurally normal heart was 38.1 ± 12.1 years, and was significantly younger than those with cardiovascular disease requiring surgery and either remained in normal sinus rhythm or developed atrial fibrillation. Similarly, systolic and diastolic function was lower in the cardiovascular disease group that required surgery (Table 2). Table 3 demonstrates the mechanism of brain death for the cardiac donors.
Structural heterogeneities
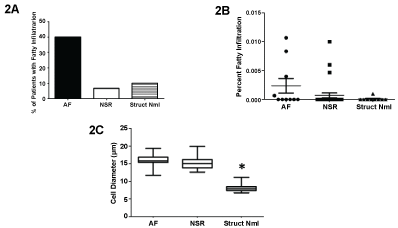
Fatty infiltration was observed in a greater number of patients requiring cardiac surgery compared to the structurally normal samples, and although there was a numerically greater percentage of patients who had evidence of fatty infiltration and developed post-operative AF, this was not significant (Figure 2A). The percent area of fatty infiltration was also not significant between groups (Figure 2B). One patient with a structurally normal heart had two adipocyte cells within a muscle bundle. Although this was viewed as abnormal, because there were no significant differences in fatty infiltration between groups, this patient was not excluded from the analysis. However, myocyte diameter was significantly lower within patients with a structurally normal heart (Figure 2C).
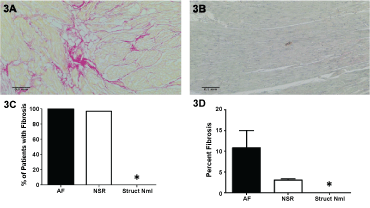
Representative images from a patient with cardiovascular disease (Figure 3A) and with a structurally normal heart (Figure 3B) demonstrated dramatic differences. The red-interdigitating strands of fibrosis can be seen in Figure 3A, while Figure 3B is devoid of these signs. Similarly, fibrosis was observed in 39/40 patients (Figure 3C) who required surgery, and the percentage of fibrosis was significantly lower in those with a structurally normal heart (Figure 3D) further validating that these heart were structurally normal.
K+ channel transcript expression
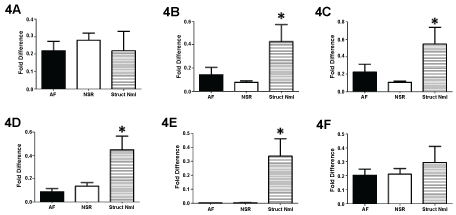
Comparison of left atrial K+ channel transcripts between those patients who required cardiac surgery and those with a structurally normal heart demonstrated significant differences (Figure 4). The expression of Kv 4.3 and Kir 2.1 were similar between groups. However, there were significant differences when comparing Kir 2.3, 3.1 3.4 and Kv 1.5 between groups. In all cases the expression of Kir 2.3, 3.1, 3.4 and Kv 1.5 was significantly greater within patients with a structurally normal heart.
Discussion
Atrial fibrillation is commonly encountered following cardiac surgery. The mechanism(s) behind this arrhythmia remain poorly understood. Our group and others have previously have demonstrated the importance of left atrial fibrosis in patients who developed AF [1-6]. Fibrosis within the left atrium is a common result from sustained hypertension, diastolic dysfunction and resulting atrial dilation. However, this is only one potential change to the left atrium which could account for the development of post-operative AF. Ion channel remodeling, has long been implicated in the development of post-operative AF, particularly potassium ion channel remodeling [9-21]. The potassium currents primarily determine the action potential duration. Further, a decreased action potential duration and lower effective refractory period are conditions commonly associated with the development of atrial fibrillation [10-12]. These conditions, however, would result only during an increased expression of K+ channel transcripts. It is therefore, interesting that we found that those patients who had cardiovascular disease had a lower expression of K+ channel transcripts compared to the structurally normal heart. We are not the first group to demonstrate this effect [10,11]. Comparison of human tissue from human patients with and without AF, demonstrated lower Kv 1.5 expression from AF patients [13]. Further, comparison of right atrial samples from patients with and without heart failure (a disease commonly associated with AF) demonstrated lower K+ channel expression within the heart failure cohort [12]. Some of these discrepancies may relate to the degree of fibrosis between groups. Changing the left atrial cell population to more fibroblasts and less myocytes may in part be reflective of decreased expression of K+ channel transcripts and would account for these differences. Particularly, myofibroblasts often activated during heart failure and chronic cardiovascular disease may increase in proportion therefore altering the overall expression of different ion channels.
Unfortunately, there were no right atrial biopsies available for examination from the structurally normal heart to determine if the left to right atrial gradients of K+ channel transcripts remain in the structurally normal heart. Although portions of the left atrium such as the appendage are often discarded during cardiectomy, use of the either the biatrial or bicaval approach to orthotopic heart transplant requires all of the right atrial tissue from the tissue donor, for the anastomosis.
Limitations
Left atrial samples were obtained following cardiectomy at the time of transplant, and therefore, the amount of tissue available for study was limited. In addition, samples were unavailable from the right atrium. Further the lack of tissue limited protein validation, and the timing and location of cardiectomy outside of our institution prohibited patch clamp data which could have validated differences in K+ channel transcripts. Last, those patients without cardiovascular disease were significantly younger, and we were unable to account for any differences between groups based upon age.
Conclusion
We demonstrate that the incidence of structural heterogeneities and differences in K+ channel transcript expression are significantly different when comparing patients with a structurally normal heart to those requiring cardiac surgery. This suggests that left atrial changes may be present in all patients with cardiovascular disease, and that these changes may be partly responsible for the development of post-operative AF. Further study, on a larger cohort of patients may be warranted.
References
- Swartz MF, Fink GW, Lutz CJ, et al. (2009) Left-versus-right atrial difference in dominant frequency, K (+) channel transcripts, and fibrosis in patients developing atrial fibrillation following cardiac surgery. Heart Rhythm 6: 1415-1422.
- Bessissow A, Khan J, Devereaux PJ, et al. (2015) Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J Thromb Haemost 13: S304-S312.
- Raiten JM, Ghadimi K, Augoustides JG, et al. (2015) Atrial fibrillation after cardiac surgery: Clinical update on mechanisms and prophylactic strategies. J Cardiothorac Vasc Anesth 29: 806-816.
- Filardo G, Pollock BD, da Graca B, et al. (2017) Underestimation of the incidence of new-onset post-coronary artery bypass grafting atrial fibrillation and its impact on 30-day mortality. J Thorac Cardiovasc Surg 154: 1260-1266.
- Swartz MF, Fink GW, Sarwar M, et al. (2012) Elevated pre-operative serum peptides for collagen I and III synthesis result in post-surgical atrial fibrillation. J Am Coll Cardiol 60: 1799-1806.
- Dobrev D, Wettwer E, Kortner A, et al. (2002) Human inward rectifier potassium channels in chronic and postoperative atrial fibrillation. Cardiovasc Res 54: 397-404.
- Kovoor P, Wickman K, Maguire CT, et al. (2001) Evaluation of the role of IKACh in atrial fibrillation using a mouse knockout model. Journal of the American College of Cardiology 37: 2136-2143.
- Zaritsky JJ, Redell JB, Tempel BL, et al. (2001) The consequences of disrupting cardiac inwardly rectifying K+ current (IK1) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol 533: 697-710.
- Finet JE, Rosenbaum DS, Donahue JK (2009) Information learned from animal models of atrial fibrillation. Cardiol Clin 27: 45-54.
- Dobrev D, Friedrich A, Voigt N, et al. (2005) The G protein-gated potassium current I (K, Ach) Is constitutively active in patients with chronic atrial fibrillation. Circulation 112: 3697-3706.
- Brundel BJ, Van Gelder IC, Henning RH, et al. (2001) Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation 103: 684-690.
- Pandit SV, Workman AJ (2016) Atrial electrophysiological remodeling and heart failure. Clinc Med Insights Cardiol 10: 41-46.
- Van Wagoner DR, Pond AL, McCarthy PM, et al. (1997) Outward K current densities and Kv 1.5 expression are reduced in chronic human atrial fibrillation. Circ Res 80: 772-781.
- Lee YS, Hwang M, Song JS, et al. (2016) The contribution of ionic currents to rate-dependent action potential duration and pattern of reentry in a mathematical model of human atrial fibrillation. PLoS One 11: e0150779.
- Wijffels MC, Kirchhof CJ, Dorland R, et al. (1995) Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92: 1954-1968.
- Darbar D, Roden DM (2013) Genetic mechanisms of atrial fibrillation: impact on response to treatment. Nat Rev Cardiol 10: 317-329.
- Hibino H, Inanobe A, Furutani K, et al. (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291-366.
- Liu L, Nattel S (1997) Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol 273: 805-816.
- Remillard CV, Tigno DD, Platoshyn O, et al. (2007) Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol 292: 1837-1853.
- Mays DJ, Foose JM, Philipson LH, et al. (1995) Localization of the Kv1.5 K + channel protein in explanted cardiac tissue. J Clin Invest 96: 282-292.
- Franqueza L, Valenzuela C, Eck J, et al. (1999) Functional expression of an inactivating potassium channel (Kv4.3) in a mammalian cell line. Cardiovasc Res 41: 212-219.
Corresponding Author
Michael F Swartz, PhD, Department of Surgery, Strong Memorial Hospital, University of Rochester Medical Center, Box Surg/Cardiac, 601 Elmwood Ave Rochester, New York 14642, USA, Tel: 585-275-2735, Fax: 585-276-1448.
Copyright
© 2017 Coello J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.