Glioblastoma and Breast Cancer: Case Report
Case Report
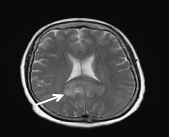
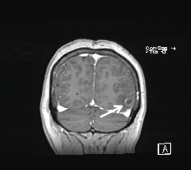
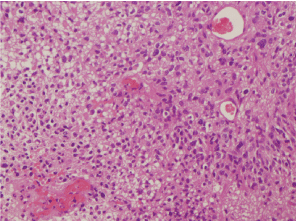
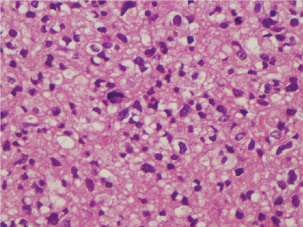
A 63-years-old woman was admitted to the hospital due to severe headache and episodes of temporospatial disorientation. She was diagnosed of infiltrating ductal carcinoma of the left breast pT2N0M0 eighteen years before, treated with surgery and tamoxifen for five years. A CT-body was performed, and several brain lesions compatible with metastasis were found. In the brain-MRI, these lesions were confirmed (Figure 1 and Figure 2). Left temporal craniotomy was performed for the removal of a cortico-subcortical lesion and histological confirmation. The histological diagnosis was glioblastoma (Figure 3 and Figure 4). The patient died one month later.
Discussion
Breast cancer survivors are at a high risk for developing second neoplasm due to an inherited predisposition or treatment impact [1].
The rate of a contralateral second primary breast cancer is around 0.5 to 1% per year [1,2], which means that 5 to 10 percent of women who have had one breast cancer will develop a second one during the 10 to 15 years following treatment of the initial cancer. The risk is similar in the preserved breast as in the contralateral breast.
Disease relapse often occurs within two to five years of the initial therapy, but an important number of second primary breast cancer occur later, necessitating long-term surveillance [3,4].
Less than 10% of all breast cancer are associated with germline genetic mutations [5]; most of them are associated with mutations in BRCA1 and BRCA2, causing hereditary breast and ovarian cancer syndrome (HBOC). Breast cancer can also be related to mutations in the TP-53 and PTEN genes, associated then to other hereditary cancer syndromes, such as Li-Fraumeni and Cowden syndromes.
Women with HBOC syndrome have markedly elevated risks of breast and ovarian cancer [5], with a lifetime risk of breast cancer of 50 to 85% and a 15-40% chance of developing ovarian cancer.
Both chemotherapy and radiotherapy may be responsible to second tumours in patients with history of breast cancer [6].
Radiotherapy has been associated with contralateral breast cancers and non-breast primary malignancies, such as sarcomas, lung cancer, oesophageal cancer and leukaemia [6-9].
The absolute magnitude of the risk for a post irradiation sarcoma is small [10]; angiosarcoma is the most frequent [11,12], presented as multiple reddish and purple nodules or areas of skin discoloration.
In three separate reports, a major risk of lung cancer has been described in breast cancer patients receiving radiotherapy. The relative risk was between 2 and 2.8 in those patients who survived 10 or more years from diagnosis [13-15]. The incidence of lung cancer is related to smoking and the volume of lung in the irradiated field [16,17].
Oesophageal cancer risk has also been associated with history of radiotherapy, but with the modern techniques, the oesophagus is excluded from the treated volume [18].
The risk of acute nonlymphocytic leukemia is related to the volume of bone marrow in the field, the total RT dose and the concomitant use of chemotherapy [19,20].
Radiotherapy is associated with a slight risk of contralateral breast cancer. Clarke, et al. [21] published in 2005 a meta-analysis in which the annual odds ratio for contralateral breast cancer for irradiated compared to nonirradiated women was 1.18 (p = 0.002).
Many chemotherapeutic agents are carcinogenic. Some of the agents usually use in the adjuvant setting are well established causes of myelodysplastic syndrome and treatment-related acute myeloid leukemia, such as alkylating agents (e.g. Cyclophosphamide) and topoisomerase II inhibitors (e.g. Doxorubicin).
Tamoxifen has been associated with an increased risk for endometrial cancer (uterine sarcoma and carcinosarcoma than for endometrial adenocarcinomas).
According to the literature, the association between breast cancer and glioblastoma is not as common as the association between breast cancer and other primary malignant tumours. The biggest series was published in 2005 by Piccirilli, et al. [22] with 11 patients. The mean age at breast cancer diagnosis was 42.9 years, and the average time between the breast cancer diagnosis and the clinical onset of GBM was 18 years.
PTEN is a tumour suppressor gene located on the 10q23 chromosomal region. It has been indicated as a possible common genetic origin of breast cancer and GBM. As noted previously, PTEN mutations are responsible for Cowden's disease, which is characterized by an increased risk for breast cancer. PTEN mutations have also been found in glioblastomas [23]. Other mutations, such as deletion on chromosome 18p, have been described in GMB and breast cancer [24].
Other molecular alterations may be shared in the malignant transformation of breast and glial tumours, such as overexpression of HER-2 [25].
GBM are rarely multicentric [26]. Multicentric GBM associated with other primary cancers is extremely rare; to our knowledge, only one patient with multicentric GBM and breast cancer has been reported [27].
We also wanted to emphasize the importance of histological confirmation of the lesions, even though they are suspected of metastasis by the image.
References
- Schell SR, Montague ED, Spanos WJ Jr, et al. (1982) Bilateral breast cancer in patients with initial stage I and II disease. Cancer 50: 1191-1194.
- Chaudary MA, Millis RR, Hoskins EO, et al. (1984) Bilateral primary breast cancer: a prospective study of disease incidence. Br J Surg 71: 711-714.
- Emens LA, Davidson NE (2003) The follow-up of breast cancer. Semin Oncol 30: 338-348.
- Kattlove H, Winn RJ (2003) Ongoing care of patients after primary treatment for their cancer. CA Cancer J Clin 53: 172-196.
- Foulkes WD (2008) Inherited susceptibility to common cancers. N Engl J Med 359: 2143-2153.
- Berrington de Gonzalez A, Curtis RE, Gilbert E, et al. (2010) Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer 102: 220-226.
- Schaapveld M, Visser O, Louwman MJ, et al. (2008) Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol 26: 1239-1246.
- Brown LM, Chen BE, Pfeiffer RM, et al. (2007) Risk of second non-hematological malignancies among 376,825 breast cancer survivors. Breast Cancer Res Treat 106: 439-451.
- Galper S, Gelman R, Recht A, et al. (2002) Second nonbreast malignancies after conservative surgery and radiation therapy for early-stage breast cancer. Int J Radiat Oncol Biol Phys 52: 406-414.
- Yap J, Chuba PJ, Thomas R, et al. (2002) Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys 52: 1231-1237.
- Young RJ, Brown NJ, Reed MW, et al. (2010) Angiosarcoma. Lancet Oncol 11: 983-991.
- Lahat G, Dhuka AR, Hallevi H, et al. (2010) Angiosarcoma: clinical and molecular insights. Ann Surg 251: 1098-1106.
- Inskip PD, Stovall M, Flannery JT (1994) Lung cancer risk and radiation dose among women treated for breast cancer. J Natl Cancer Inst 86: 983-988.
- Neugut AI, Robinson E, Lee WC, et al. (1993) Lung cancer after radiation therapy for breast cancer. Cancer 71: 3054-3057.
- Prochazka M, Granath F, Ekbom A, et al. (2002) Lung cancer risks in women with previous breast cancer. Eur J Cancer 38: 1520-1525.
- Kaufman EL, Jacobson JS, Hershman DL, et al. (2008) Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol 26: 392-398.
- Deutsch M, Land SR, Begovic M, et al. (2003) The incidence of lung carcinoma after surgery for breast carcinoma with and without postoperative radiotherapy. Results of National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials B-04 and B-06. Cancer 98: 1362-1368.
- Salminen EK, Pukkala E, Kiel KD, et al. (2006) Impact of radiotherapy in the risk of esophageal cancer as subsequent primary cancer after breast cancer. Int J Radiat Oncol Biol Phys 65: 699-704.
- Welte B, Suhr P, Bottke D, et al. (2010) Second malignancies in high‑dose areas of previous tumor radiotherapy. Strahlenther Onkol 186: 174-179.
- Cole M, Strair R (2010) Acute myelogenous leukemia and myelodysplasia secondary to breast cancer treatment: case studies and literature review. Am J Med Sci 339: 36-40.
- Clarke M, Collins R, Darby S, et al. (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366: 2087-2106.
- Piccirilli M, Salvati M, Bistazzoni S, et al. (2005) Glioblastoma multiforme and breast cancer: report on 11 cases and clinico-pathological remarks. Tumori 91: 256-260.
- Li J, Yen C, Liaw D, et al. (1997) PTEN, a putative protein tyrosine phosphatise gene mutated in human brain, breast and prostate cancer. Science 275: 1943-1947.
- Tran Y, Benbatoul K, Rempel S, et al. (1998) Novel regions of allelic deletion on chromosome 18p in tumors of the lung, brain and breast. Oncogene 17: 3499-3505.
- Koka V, Potti A, Forseen SE (2003) Role of her-2/neu overexpression and clinical determinants of early mortality in glioblastoma multiforme. Am J Clin Oncol 26: 332-335.
- Jawahar A, Weilbaecher C, Shorter C, et al. (2003) Multicentric glioblastoma multiforme determined by positron emisión tomography: a case report. Clin Neurol Neurosurg 106: 38-40.
- Sarina E, Huse J, Mason B, et al. (2006) Multicentric glioblastoma multiforme in a patient with BRCA-1 invasive breast cancer. The Breast Journal 12: 470-474.
Corresponding Author
López González A, Medical Oncology, University Assistance Complex of León, Spain.
Copyright
© 2017 López Flores M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.