Development and Validation of Potentiometric ZnO Nanorods Modified Ion Selective Electrode for Determination of Gemifloxacin in Pharmaceutical Formulation
Abstract
Potentiometric method for determination of Gemifloxacin (GEMI) by ion selective electrode based on ZnO nanorods incorporation with HPβ-CD as sensing ionophore and (KTFPB) potassium tetrakis- (3,5(Triflouromethyl) Phenyl Borate) ion as anionic site (additive) in Polyvinyl Chloride (PVC) membrane, without inner reference solution was developed. The sensor shows 'nearly Nernstian response over a concentration range (0.5-10000 µM) with a slope of 33.65 mv decade-1 of concentration with a Limit of Detection (LOD) 0.1500 µM. The electrode exhibits a fast dynamic response of 2 s for a period of 6 months without significant change in its characteristics with excellent stability and sensitivity toward inorganic species. The method is accurate and precise as indicated by the mean recoveries 106.43% with RSD less than 2%. The proposed method was successfully applied for the determination of GEMI in pharmaceutical formulations.
keywords
Potentiometric, Ion selective electrode, Zno nanorode, GEMI, PVC membrane
Introduction
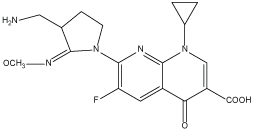
Gemifloxacin Mesylate (GEMI), chemically known as [(R, S)-7-[(4Z)-3 (aminomethyl)-4-(methoxyimino)-1-pyrrolidinyl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1, 8-naphthyridin-3-carboxylic acid mesylate] Figure 1. Its fourth generation fluoroquinolone antibacterial agent having affinity towards bacterial topoisomerase IV, It has broad spectrum of activity against gram-positive and gram-negative bacteria [1]. GEMI has shown potent activity against other major pathogens involved in respiratory tract infections, including Haemophilus influenza and the atypical organisms, Legionella pneumophila, Chlamydia spp, and Mycoplasma spp [2]. Furthermore, the compound has shown potent activity against many organisms that cause urinary tract infections. The adverse reaction profile is similar to that of older members of this class [3]. The great bactericidal activity of GEMI is due to the presence of 4-oxo-3-carboxylic acid [4].
A number of analytical methods have been reported for the determination of GEMI in pharmaceutical formulation and biological samples. These include High Performance Liquid Chromatography (HPLC) [5], HPLC coupled with Mass Spectrometry (HPLC-MS) [6], Capillary electrophoresis [7], Gas Chromatography-Mass Spectrometry (GC-MS) [8], Spectrophotometery [9], Spectrofluorimetry [10,11], Voltammetry [12] and Chemiluminescence [13]. Most of these methods are complicated involve derivatization procedures, requires intensive instruments also it's time and labor consuming.
Potentiometric sensors are easy to miniaturize and provides a large dynamic range. In conventional ion selective electrodes, Polyvinyl Chloride (PVC) is the most commonly used matrix as the selective membrane [14]. The ion-selective membrane exhibits the selectivity with which the sensing material responds to the analyte and an electrochemical equilibrium is reached. The resulting potential difference, formed between the phases, will then be governed by the activity of this specific ion in the two solution phases [15].
Potentiometric methods, using ion selective electrodes, have found wide application [16,17] being simple analysis procedures, economical coasts, fast results, applicable over a wide range of concentrations, applicability to various drug forms, with applicability to turbid and colored solutions, preciseness and offering enough selectivity towards the drug in the presence of various pharmaceutical excipients.
Al-Mohaimeed, et al. [16] developed potentiometric sensors using different such as ion-pairing agents Phosphotungstic Acid (PTA), Phosphomolybdic Acid (PMA) and Ammonium Reineckate Salt (ARS). The sensors exhibit good selectivity for GEMI with respect to some inorganic cations, amino acids and some pharmacologically related compounds [16]. Abo-talib demonstrated PVC membrane sensors for the determination of GEMI. The sensors are based on the use of the ion association complexes of GEMI cation with ammonium reineckate counter anions as ion exchange sites in the PVC matrix. The membranes incorporate ion association complexes of GEMI with dibutylsebathete, dioctylphthalate, nitrophenyl octyl ether. The proposed sensors were successfully applied for determination of GEMI in bulk powder, pharmaceutical formulation, and biological fluids [17].
Research on ZnO nanostructures have been fueled by the observation that the material properties depend not only on the composition but also on the size and shape [18]. Recently, ZnO nanowires, nanorods and nanotubes have gained much attraction due to their high surface-to-volume ratio which makes them extremely sensitive to minute surface changes. In addition, one-dimensional ZnO nanostructures are promising for sensing due to their ease to grow vertically on almost any substrate [18-20].
The aim of this work is to develop and validate a simple, sensitive, rapid and miniaturized potentiometric ZnO nanorods based ion selective electrode without inner reference solution for the determination of GEMI in pharmaceutical formulations. Ion selective electrode consisted of PVC, dibutyl phthalate, 2-Hydroxypropyl)-β-cyclodextrin (HPβ-CD) and Potassium Tetrakis (3,5(TriFlouromethyl)Phenyl) Borate (KTFPB) as matrix, plasticizer, sensing ionophore and anionic additive, respectively were used to develop the sensor.
Materials and Methods
Chemicals and reagent
Gemifloxacin was obtained from (98%, Bayer AG, Leverkusen Germany), HPβCD (ionophore), Potassium Tetrakis (3,5(TriFlouroMethyl)Phenyl) Borate (KTFPB) (additive), PVC (high molecular weight), dibutyl phthalate (a plasticizer), Zinc Acetate (ZnAc), Hexamethylenetetramine (HMTA) ware purchased from sigma Aldrich (St.Louis, USA), silver wire (0.3 mm diameter), Na2HPO4, H3PO4, KOH, acetone, isopropanol, Tetrahydrofuran (THF), methanol, (all solvent with HPLC grade), Factive tablets (320 mg GEMI per tablet) [LG life science Ltd, Kore lisansiyla Abdilbrahim ilacsan.VeTic.A.S. Maslak/Istanbul 3.5.2016, 210/86], Deionized water.
Instrument and apparatus
pH/mv meter (PHS-3E) (China), Ag/AgCl reference electrode (Ω metrohm.Autolab, inner and outer filling by KCl 3 M. (Netherlands), sensitive balance, magnetic hot plate, thermometer, oven, SEM (Zeiss Evo LS 10.Germany).
Seed and growth ZnO nanorods
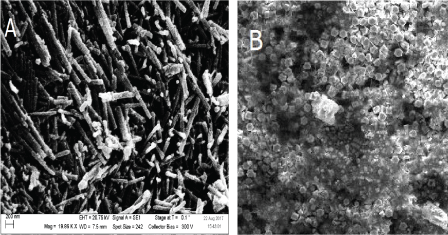
ZnO nanorods were grown by low temperature aqueous chemical method [19]. A silver wire (0.3 mm) was cut in the length of 5 cm and cleaned by acetone and isopropanol for 2 min in each solution followed by rinsing with deionized water and left to dry at room temperature. The silver wire was immersed three times in a seed solution prepared by mixing alcoholic solutions of KOH added drop wise to heated, stirred 0.03 M of zinc acetate the resulting solution was kept under stirring for 2 hours at 60 ℃ prior dipping , the wires was left to dry at room temperature. The ZnO was grown by suspending the pre-coated Ag wire in aqueous solutions contains 0.025 M ZnAc with equimolar concentration of HMTA. The beaker was placed in preheated oven at 70 ℃ to 5 hours. The wires were cooled down, washed by deionized water and left to dry over night. The ZnO nanorods were characterized by SEM (Zeiss Evo LS 10, Germany) Figure 2.
Coating ZnO nanorods with Ion selective membrane
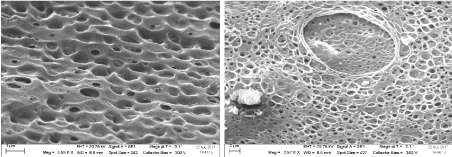
ZnO nanorods were coated by ion selective membrane by mixing 33% PVC, 66% DBP plasticizer, 1.2% HPβ-CD (ionophore), 0.4% KTFPB (ionic additive) in 5 GEMI-ZnO-seml THF. The ZnO coated wires was dipped twice into a prepared solution, after each dip the electrode was left to dry at room temperature, then the electrode was conditioned into 1 × 10-3 M of GEMI standard solution for 24 hour prior to use. The membrane was characterized by SEM (Zeiss Evo LS 10, Germany) Figure 3.
Standard drug solutions
Stock standard solutions 0.01 M GEMI (Mw = 389.381 g/mol) was prepared by dissolving accurate weight in 5 mL of 0.1 M NaOH and the volume was completed by deionized water, this solution was kept in the dark at 4 ℃. Working solutions ranging 0.5-10000 µM were prepared by serial dilution of the stock solution by deionized water. The testing series was prepared by adding adequate amount of (0.2 M) phosphate buffer (H3PO4/Na2HPO4) [Ph = 3; 0.2 M] and desired volume of drug stock solution and the volume completed to mark by deionized water.
Preparation of GEMI sample solution
5 tablets of Factive (contain 320 mg GEMI per tablet) were weighed and the average weight was determined then it grounded into fine powder using mortar. Solution with 0.001 M was prepared by taken accurate weight of the powder dissolved by 5 mL of 0.1 M NaOH and 5 mL of phosphate buffer (H3PO4/Na2HPO4) [pH 3, 0.2 M] were added, the volume was completed to 250 mL by deionized water. The resulting solution was filtrated through filter paper.
Electrochemical measurements
In a complete potentiometric cell, the GEMI -ZnO-selective electrode was used in conjunction with Ag/AgCl reference electrode (inner and outer filling by KCl 3 M). The electrochemical potential between the GEMI-ZnO-selective electrode as cathode and Ag/AgCl reference electrode (Ω metrohm.Autolab, inner and outer filling by KCl 3 M) as anode was measured with pH/mv meter (PHS-3E).
GEMI.TFPB - PVC || Test solution || Ag/AgCl (3 M.KCl)
The measured potential was plotted against the logarithm of drug concentration. The electrode was washed with deionized water blotted with tissue paper between measurements.
Results and Discussions
Optimization conditions
Effect of pH
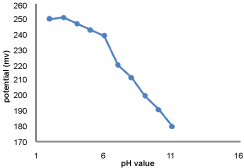
The effect of pH on the potential response of the GEMI-ZnO-ISE was investigated using 1 × 10-4 M solutions in pH range of 2.0-11.0 using Na2HPO4/H3PO4 (0.2 M) as a buffer solution. The potential readings corresponding to different pH values were recorded and plotted using the proposed electrode. Increasing in electrode potential was observed in pH range from 2 to 3 and decreased from pH 4 to 11, Figure 4. These result suggested that the inclusion complex of GEMI and HPβ-CD was suitable in acidic media because GEMI containing primary amine group that capable to bind with protons presents in acidic media resulting positively charged GEMI ion, which therefore can attracted by anionic tetraphenyl borate group present in the additive (KTFPB) and hence facilities the inclusion between GEMI and HPβ-CD [16,17,21].
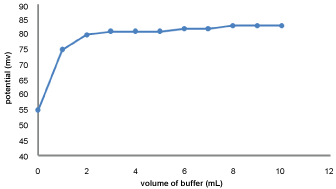
Effect of volume of buffer
The effect of volume of buffer on the potential response of the GEMI -ZnO-ISE was studied using 1 × 10-4 M solutions in the range of (0-10) mL using Na2HPO4/H3PO4 [pH 3; 0.2 M]. It was found that the potential increased when buffer adding to GEMI solution without buffer and the potential remains constant with adding extra volume of buffer as shown in Figure 5.
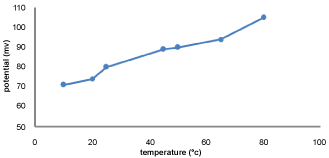
Effect of temperature
The effect of temperature on the potential response of the GEMI-ZnO-ISE was studied using 1 × 10-4 M solutions at the range of temperature (10-80) ℃ using thermometer presented in Figure 6. It reveals that the potential increased with increasing temperature of drug solution this could be attributed to potentiometric measurements is equilibrium controlled [22], thus increasing solution temperature is resulting faster equilibrium between the electrode surface and GEMI solution.
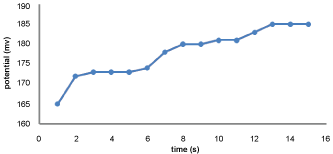
Response time
The response time of potential of the GEMI-ZnO-ISE was studied using 1 × 10-4 M solutions in a period from 0 to 15 second. The potential readings corresponding to time were recorded and plotted using the proposed electrode in Figure 7. The sensor display very fast and response within 2 second.
Electrode composition
The electrode shows linear Nernestian response over a wide range of concentration 0.5-10000 µM, stable, sensitive and very fast response. This attributed to electrode compositions. The ZnO nanorods increased the surface area for distribution of the membrane compared if it directly attached to silver wire, thus increased the sensitivity of the electrode and decreases the response time [18]. HPβ-CD is used as sensing ionophore, the most important property of CDs is their ability to form supramolecular inclusion complexes with many appropriately sized organic ions and molecules in aqueous, non-aqueous and mixed media [11]. The driving forces for the complexation are non-covalent, including Van der Waals forces and directed hydrogen bonding. Water molecules in CD cavity are displaced by more hydrophobic guest molecules present in the solution to attain a non-polar/non-polar association and decrease of CD ring strain resulting in a more stable lower energy state [23]. On constructing an ISE, the amount of the sensing ionophore in the electrode matrix should be sufficient to obtain reasonable complexation at the electrode surface that is responsible for the electrode potential [24,25].
The function of KTFPB as lipophilic ionic additives is to promote the interfacial ion exchange kinetics and decrease the electrode resistance through enhancing the ionic mobility in the electrode matrix. The response of ISEs containing ionic sites can be distinguished whether the incorporated ionophore acts as an electrically charged or uncharged carrier [26,27].
Statistical data
The analytical methods were validated with respect to linearity, Limit of Detection (LOD), Limit of Quantification (LOQ) and precision according to ICH [28,29].
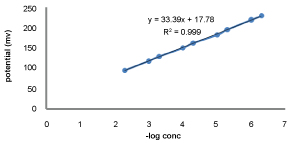
Calibration curve and statistical data for GEMI
The measuring range of a potentiometric sensor was the linear part of the calibration graph as shown in Figure 8. The critical response coated wire sensor electrodes were determined and the results were summarized in Table 1. LOD and LOQ were determined using the formula: LOD or LOQ = K.SD a/b, where K = 3.3 for LOD and 10 for LOQ, SDa is the standard deviation of the intercept, and b is the slope. the values of LOD and LOQ were found to be 0.15 and 0.4546 µM respectively. The sensor show nearly Nernestian response over the concentration range 0.5-10000 µM of the GEMI standard solution. Calibration graph slope for sensor electrode were 33.65 mV decade-1. The electrodes exhibited a fast dynamic response of 2 s for a period for more than 6 months without significant change in the electrodes parameters.
Accuracy and precision of the potentiometric method
The accuracy and precision of the proposed method was determined at three concentration levels of GEMI by apply three replicate samples of each concentration. The standard deviations for the results did not exceed 2% are listed in Table 2, indicating high reproducibility of the results and precision of the method. This good level of precision was suitable for quality control analysis of GEMI and in the pharmaceutical formulations.
Robustness of potentiometric method for GEMI
Robustness was examined by evaluating the influence of small variation in the method variables on its analytical performance. In these experiments, one parameter was changed, whereas the others were kept unchanged, and the recovery percentage was calculated each time. It was found that small variables did not significantly affect the procedures, recovery values were shown in Table 3.
Analysis of pharmaceutical formulations
Proposed methods were applied to the pharmaceutical formulations and indicate the high accuracy of the proposed method for determination of GEMI. The proposed method has advantage of being virtually free from interferences by excipients. The percentage was 106.43 ± 0.51.
Recovery study of the potentiometric method
To a fixed amount of the drug in the dosage form and pure drug (the standard) were added at five different levels and the total was found by the proposed method each test was performed in triplicate. Table 4 revealing good accuracy and no interference from excipients. Recovery was calculated as the amount found/amount taken × 100.values are mean ± R.S.D. for three determinations.
The sensitivity
The sensitivity was tested by adding some inorganic salts and diluted acids and bases, it was found that the electrode shows excellent sensitivity toward testing species with RSD less than 2%.
Reproducibility
The electrode response shows excellent repeatability during analysis and very stable response with intraday RSD did not exceeded 2%, and inters day analysis with RSD less than 5%.
Conclusion
It can be concluded that GEMI-ZnO-ISE offers a viable technique for the direct determination of GEMI in pharmaceutical preparations. The sensor allows simple, rapid, and reproducible determination over a wide linear range of concentration with the same sensitivity without the need of complex sample manipulations. The sensor exhibits a good selectivity towards the drug in the presence of various pharmaceutical recipients, long life time and time-labor saving.
The procedure avoid the usual pretreatment steps necessary for GEMI assays and presents some general advantages over common chromatographic and spectroscopic procedure, it makes use of less sophisticated equipments (there for being easier to operate and providing lower cost of analysis) and surpasses color and turbidity problems associated with suspensions and colloids.
Sensor accomplished LOD and LOQ of 0.15 µM, 0.4546 µM, respectively with a fast response time of less than 5 seconds.
Acknowledgment
The authors gratefully acknowledge Prof. Omer Nur from (LinkÖping University, NorrkÖping, Sweden) for providing facilities to accomplish this work and Dr. Manal Siyam for analysis samples by SEM in Naturkundi Museum laboratories-Berlin-Germany.
References
- Charan DC, Satyabrata S (2011) Simple and rapid specterophotometric estimation of gemifloxacin mesylate in bulk andtablet formulations. International Journal of PharmTech Research 3: 133-135.
- Hannan P, Woodnutt G (2000) In vitro activity of gemifloxacin against human mycoplasmas. J Antimicrob Chemother 45: 367-369.
- Thakre YM, Choudhary MD (2011) Synthesis, characterization and evaluation of derivative of Ciprofloxacin (1-cyclopropyl-6-fluoro-4-oxo-7-[4-(phenyl carbonyl) piperazin-1-yl]-1, 4-dihydroquinoline-3-carboxylic acid) and their complexes. J Chem Pharm Res 3: 390-398.
- Szejtli J (1988) Cyclodextrin Technology. In: Kluwer Academic Publishers, Dordrecht, Netherlands, 1-78.
- Sugumaran M, Jotheeswari D (2011) RP-HPLC method for the determination of gemifloxacin mesylate in bulk and pharmaceutical formulation. Int J Pharm Sci Rev Res 6: 18-20.
- Doyle E, Fowles SE, McDonnell DF, et al. (2000) Rapid determination of gemifloxacin in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Sci Appl 746: 191-198.
- Elbashir AA, Saad B, Salhin A, et al. (2008) Validated Stability Indicating Assay of Gemifloxacin and Lomefloxacin in Tablet Formulations by Capillary Electrophoresis. J Liq Chromatogr Relat Technol 31: 1465-1477.
- Robledo VR, Smyth WF (2008) A study of the analytical behaviour of selected new molecular entities using electrospray ionisation ion trap mass spectrometry, liquid chromatography, gas chromatography and polarography and their determination in serum at therapeutic concentrations. Anal Chim Acta 623: 221-230.
- Ebraheem SAM, Elbashir AA, Aboul-Enein HY (2011) Spectrophotometric methods for the determination of gemifloxacin in pharmaceutical formulations. Acta Pharm Sinica B 1: 248-253.
- Tekkeli SEK, Onal A (2011) Spectrofluorimetric Methods for the Determination of Gemifloxacin in Tablets and Spiked Plasma Samples. J Fluoresc 21: 1001-1007.
- Dsugi NFA, Elbashir AA, Suliman FEO (2015) Supra molecular interaction of gemifloxacin and hydroxyl propyl β-cyclodextrin spectroscopic characterization, molecular modeling and analytical application. Spectrochim Acta A Mol Biomol Spectrosc 151: 360-367.
- Zha F, Zhao W, Xiong W (2012) Chemiluminescence determination of gemifloxacin based on diperiodatoargentate (III)-sulphuric acid reaction in a micellar medium. Luminescence 28: 108-113.
- Mahajan RK, Kaur I, Sharma V, et al. (2002) Sensor for Silver(I) Ion Based on Schiff-base-p-tertbutylcalix[4]arene. Sensors 2: 417-423.
- Mascini M, Pallozzi F (1974) Selectivity of neutral carrier-pvc membrane electrodes. Anal Chim Acta 73: 375-382.
- Ganjali MR, Norouzi P, Rezapour M (2006) Encyclopedia of Sensors, Potentiometric Ion Sensors. American Scientific Publisher 8: 197-288.
- Al-Mohaimeed M, Al-Tamimi SA, Alarfaj NA, et al. (2012) New Coated Wire Sensors for Potentiometric Determination of Gemifloxacin in Pure Form, Pharmaceutical Formulations and Biological Fluids. Int J Electrochem Sci 7: 12518-12530.
- Abo-talib NF (2013) Ion Selective Electrodes for Stability-Indicating Determination of Gemifloxacin Mesylate. Anal Bioanal Electrochem 5: 74-86.
- Qin Y, Wang XD, Wang ZL (2008) Microfibre-nanowire hybrid structure for energy scavenging. Nature 451: 809-813.
- Vayssieres L (2003) Growth of Arrayed Nanorods and Nanowires of ZnO from Aqueous Solutions. Advanced Materials 5: 464-466.
- Anees A, Ansari M, Alhoshan MS, et al. (2010) Nanostructured Metal Oxides Based Enzymatic Electrochemical Biosensors. In Tech Rijeka 1: 23-46.
- Maha ET, Sawsan R, Magda EM, et al. (2010) Construction and Optimization of Selective Membrane Electrodes for Determination of Doxepin Hydrochloride in Pharmaceutical Preparations and Biological Fluids. J Kor Chem Soc 54: 198-207.
- Zhang J, Harris AR, Cattrall RW, et al. (2010) Voltammetric Ion-Selective Electrodes for the Selective Determination of Cations and Anions. Anal Chem 82: 1624-1633.
- Suliman FEO, Elbashir AA, Schmitz OJ (2015) Study on the separation of ofloxacin enantiomers by hydroxyl-propyl-β-cyclodextrin as a chiral selector in capillary electrophoresis: a computational approach. Journal of Inclusion Phenomena and Macrocyclic Chemistry 83: 119-129.
- Elbashir AA, Dsugi NFA, Aboul-Enein HY (2014) Supramolecular Study on the Interaction Between Ofloxacin and Methyl β-Cyclodextrin by Fluorescence Spectroscopy and its Analytical Application. J Fluoresc 24: 355-361.
- Khaled E, Hassan HNA, Mohamed GG, et al. (2010) β-Cyclodextrin-based potentiometric sensors for flow-injection determination of acetylcholines. Int J Electrochem Sci 5: 448-458.
- Bakker E, Pretsch E (1995) Lipophilicity of tetraphenylborate derivatives as anionic sites in neutral carrier-based solvent polymeric membranes and lifetime of corresponding ion-selective electrochemical and optical sensors. Anal Chim Acta 309: 7-17.
- Hansen AJ (1981) Extracellular ion concentrations during cerebral ischemia. In: Zeuthen T, The Application of Ion-Selective Microelectrodes. Elsevier, New York, 239-254.
- Topic Q2 (R1) (2005) Validation of analytical procedures: Text and morphology. International Conference of Harmonization (ICH).
- (1995) ICH-Q2A guideline for industry March. Text on validation of analytical procedures.
Corresponding Author
Professor Abdalla A Elbashir, Department of Chemistry, Faculty of Science, University of Khartoum, Sudan.
Copyright
© 2017 Idress MO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.