The Utility of Spermidine Serum Levels as a Biomarker of Alzheimer's Disease a Pilot Study
Abstract
Background/Aims
There is a close link between iron and polyamine biosynthesis and metabolism. In a recent study, we reported alterations in the serum levels of hepcidin and other iron-related proteins in Alzheimer's disease (AD) patients (Sternberg, et al., 2017). Based on these findings, this pilot study compared serum levels of one of the polyamines, Spermidine, between AD, mild cognitive impairment (MCI), and control subjects, correlating with the existing neuroimaging data.
Methods
This cross-sectional, retrospective, single-center study, used frozen serum samples of 43 AD patients, 12 MCI patients, and 21 age-matched controls, provided by the Oregon Alzheimer's Disease Center Bio-repository to measure Spermidine serum levels using enzyme-linked immunosorbent assay.
Results
MCI patients showed significantly higher mean Spermidine serum levels compared to controls (P = 0.01). Spermidine serum levels were not significantly higher in AD (P = 0.19) and pure AD (P = 0.08) participants compared to controls. A significant correlation was found between Spermidine serum levels and the values of the cognitive assessment tests, including MMSE (r = -0.705, P = 0.003), CDR (r = 0.751, P = 0.002), and CDR-SOB (r = 0.704, P = 0.007) in pure AD subgroup, but these correlations lost significance when examined for the total AD group. A significant correlation was observed between Spermidine and the chief iron regulatory protein, hepcidin, in AD participants with a more advanced disease stage, indicated by MMSE in the strata of 8-19 (P = 0.02), and CDR-SOB in the strata of 6-12 (P = 0.03).
Conclusion
Studies with larger cohort are warranted to determine the role of Spermidine in AD pathophysiology, and the utility of polyamines as biomarkers of AD diagnosis and progression.
Keywords
Iron homeostasis, Ornithine decarboxylase, Polyamine, Serum biomarker
Highlights
• We measured serum levels of one of the polyamines, spermidine, in AD, MCI, and controls
• Spermidine serum levels were significantly higher in MCI as compared to controls
• Spermidine serum levels correlated with MMSE, CDR, and CDR-SOB
Introduction
Alzheimer's disease (AD) is a chronic progressive neurological disease, where amyloid-beta (Aβ) and tau protein aggregates are among common brain pathologies [1]. The current AD diagnosis depends on the combination of clinical, functional, behavioral, and cognitive assessments tests [2] but the definitive diagnosis of AD requires pathological evaluation at the time of autopsy. In addition, between 5-30% of patients who present with mild cognitive impairment (MCI) are likely to subsequently develop Alzheimer dementia, with the risk factors for conversion being the presence of an APOE4 allele, and the volume of the hippocampus and ventricles, among others [3]. However, there are no reliable tools to identify those patients, who are likely to benefit from early intervention.
Polyamines, Spermidine and spermine, are low molecular polycarbonic polyamine essential for normal cellular function, which are involved in cell growth and proliferation, adhesion and migration, gene regulation and signaling [4]. Spermidine is synthesized from its precursor putrescine by the action of Spermidine synthase. Putrescine is produced by arginine conversion to ornithine by the action of the enzyme arginase and subsequent decarboxylation by ornithine decarboxylase (ODC). Spermidine can be then converted to spermine, by the action of spermine synthase [5].
Metabolic profiling of brains of AD subjects show increased Spermidine and spermine in frontal and parietal lobe, with a more significant increase in Spermidine compared to spermine [6]. In addition, autopsied brain of AD patients show an increase in the activity of S-adenosyl methionine decarboxylase (SAMDC), the key rate-limiting enzyme for Spermidine and spermine biosynthesis [7], as well as 70% increase in the activity of the enzyme ODC in the temporal cortex. This increase was not observed in other neurodegenerative diseases such as spinocerebellar ataxia type I [8].
A small positively charged molecule, polyamines are able to bind amyloid (A)β 1-40, promoting its aggregation as observed by NMR spectroscopy. Atomic force microscopy showed differences in the morphology of Aβ aggregates produced in the presence of polyamines, with Spermidine producing Aβ oligomers [9] known to be highly neurotoxic [10]. These studies collectively suggest a role of polyamines in modulating AD neurodegenerative processes.
Although polyamines and their metabolites have been extensively studied as biomarkers in various forms of cancer, there are no published studies to date measuring the potential of polyamines serum levels as biomarkers in AD. A search for biomarkers of AD diagnosis using metabolomics approach reported reduced serum levels of Spermidine metabolite, N-acetyl-Spermidine, correlating with AD progression [11], suggesting possible polyamines' dyshomeostasis in AD pathology.
In a recent study of AD participants [12], we showed dysregulation in the chief iron regulatory protein, hepcidin and other iron-related proteins, in AD subjects. Polyamines' biosynthesis and metabolism are known to be intimately linked to iron metabolism [13]. Therefore, dysregulation in iron-related proteins would likely impact polyamine serum levels. This pilot study aimed to determine whether serum levels of one of the polyamines, Spermidine, are altered in AD subjects and evaluate its potential as a biomarker of AD diagnosis and progression.
Materials & Methods
Population
This cross-sectional, retrospective, single-center study, was conducted on frozen serum samples provided by the Oregon Alzheimer's Disease Center Bio-repository. The samples represented 43 AD participants (30 males), age 71.3 ± 11 years, 12 MCI (8 males), age 67.3 ± 7 years, and 21 controls (10 males), age 70.4 ± 8 years. Autopsy results were available for 28 (19 males) participants which confirmed the diagnosis of pure AD in 15 (9 males) (Table 1A). The term "pure AD" refers to those subjects who present with brain pathology indicative of AD, without the presence of additional pathology related to other neurodegenerative diseases, the latter often termed "mixed dementia". The 13 remaining participants had a combination of AD/vascular dementia (n = 4), AD/Lewy body dementia (n = 5), AD/hippocampal sclerosis (2), and AD/Parkinson (2). All participants in the study (both AD and Controls) were outpatients.
Participants at the Oregon Alzheimer Disease Center Bio-repository signed informed consent for their clinical data to be used for research purposes and for their serum samples to be harvested.
Measurements
Enzyme-linked immunosorbent assay (ELISA) kit was used to measure Spermidine serum levels (My Biosource, Cat # MBS2700698, detection range 2.47 ng/ml-2515 ng/ml, and sensitivity of < 0.88 ng/ml). We also examined the correlation between Spermidine serum levels and participants' cognitive assessment test results, including mini-mental state examination (MMSE) test, clinical dementia rating (CDR), and CDR-sum of boxes (SOB); and neuroimaging data, including total CNS volume, ventricular CSF volume, hippocampal volume, subarachnoid volume, and white matter hyper-intensity volume.
Data on Aβ and iron-related proteins were available from our previous study [12], for a subgroup of 24 (17 males) participants, allowing us to correlate between Spermidine serum levels and the levels of Aβ40 and Aβ42, and between Spermidine serum levels and iron related proteins, hepcidin, ferritin, serum iron, transferrin (measured indirectly as total iron binding capacity (TIBC), and percent transferrin saturation.
Statistical analysis
Non-parametric tests were used to compare AD and the subgroups, MCI, and controls, adjusted for differences in autopsy status and post-autopsy diagnosis. Spermidine was calculated and compared between AD participants and controls, pure AD participants and controls, MCI and controls, and between AD and MCI, and P < 0.05 was indicated as statistically significant. Pearson correlation was used to test the relationship between Spermidine and cognitive assessment test results, neuroimaging data and other clinical data (Aβ and iron-related proteins). Because we were testing a priori hypotheses on Spermidine levels, rather than engaging in exploratory statistical analyses, the correlation analysis was not corrected for multiple tests.
Human subjects
The study was approved by the Internal Review Board of the State University of New York at Buffalo. The collection of data and samples was approved by the Oregon Health and Science University Internal Review Board.
Results
Table 1A presents demographics of participants. The ages of the groups did not differ significantly (all P > 0.05). In addition, 67% of AD participants, 53% of pure AD participants and 58% of MCI participants had at least one copy of the APOE4 allele; while percentages were 28% for controls (AD vs. controls P = 0.002; Pure AD vs. controls P = 0.09; MCI vs. controls P = 0.02).
Table 1B presents the severity of dementia in AD participants, pure AD participants, MCI participants, and controls, indicated by MMSE, CDR, and CDR-SOB. The data are presented as mean ± SD. MMSE scores were significantly lower in AD (20.7 ± 6, P < 0.001) and pure AD (22.7 ± 7, P < 0.001), compared to controls (29.4 ± 1.0), but MMSE did not differ significantly between MCI (28.5 ± 1) and controls (P = 0.11).
The CDR was significantly higher in AD (0.85 ± 0.5, P < 0.001) and pure AD participants (0.64 ± 0.5, P < 0.001) compared to controls (0.0 ± 0.0). CDR was also higher for MCI participants (0.18 ± 0.2) compared to controls (P = 0.009). Similarly, CDR-SOB was significantly higher for AD (5.7 ± 3, P < 0.001), pure AD (4.2 ± 3, P < 0.001), and MCI (0.45 ± 0.8, P = 0.02) participants compared to controls (0.0 ± 0.0).
Among AD participants, 81% were on acetylcholinesterase inhibitors, 18% were on Memantine, 82% on cardiovascular drugs, and 65% were on psychotropic drugs. Among controls, 85% were on cardiovascular drugs and 25% were on psychotropic drugs (Table 1C).
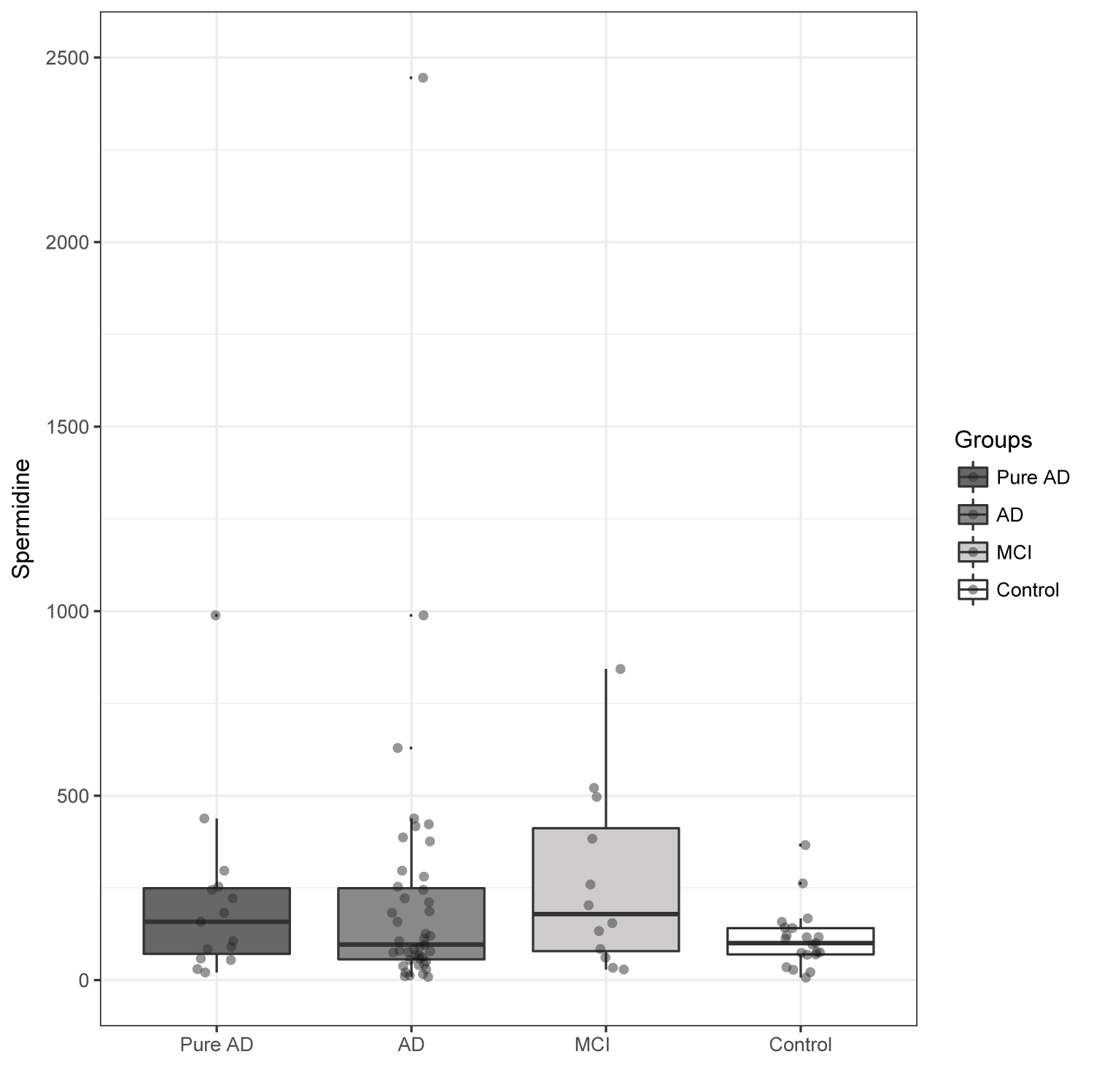
Table 2 and Figure 1 show data comparing Spermidine serum levels between AD participants, pure AD participants, MCI participants, and controls. The term "pure AD" refers to those subjects who present with brain pathology indicative of AD, without the presence of additional pathology related to other neurodegenerative diseases, the latter often termed "mixed dementia. The results of Spermidine in Participants with mixed dementia upon autopsy were analyzed as part of the total AD group. Table 2 shows the results as mean ± SD. Figure 1 shows both the mean and median in each group, adjusted for gender.
Comparison between MCI and controls showed significant differences in mean Spermidine serum levels in MCI patients (266.9 ± 249.4 ng/ml) compared to controls (112.3 ± 82.0 ng/ml, p = 0.018). However, the differences between AD (227.2 ± 395.7 ng/ml, P = 0.12) and pure AD (215.3 ± 243.2 ng/ml, P = 0.08) versus controls (112.3 ± 82.0 ng/ml), remained statistically non-significant, partly due to high inter-individual variability. No differences between MCI and AD participants were observed (P = 0.74).
Correlation Studies
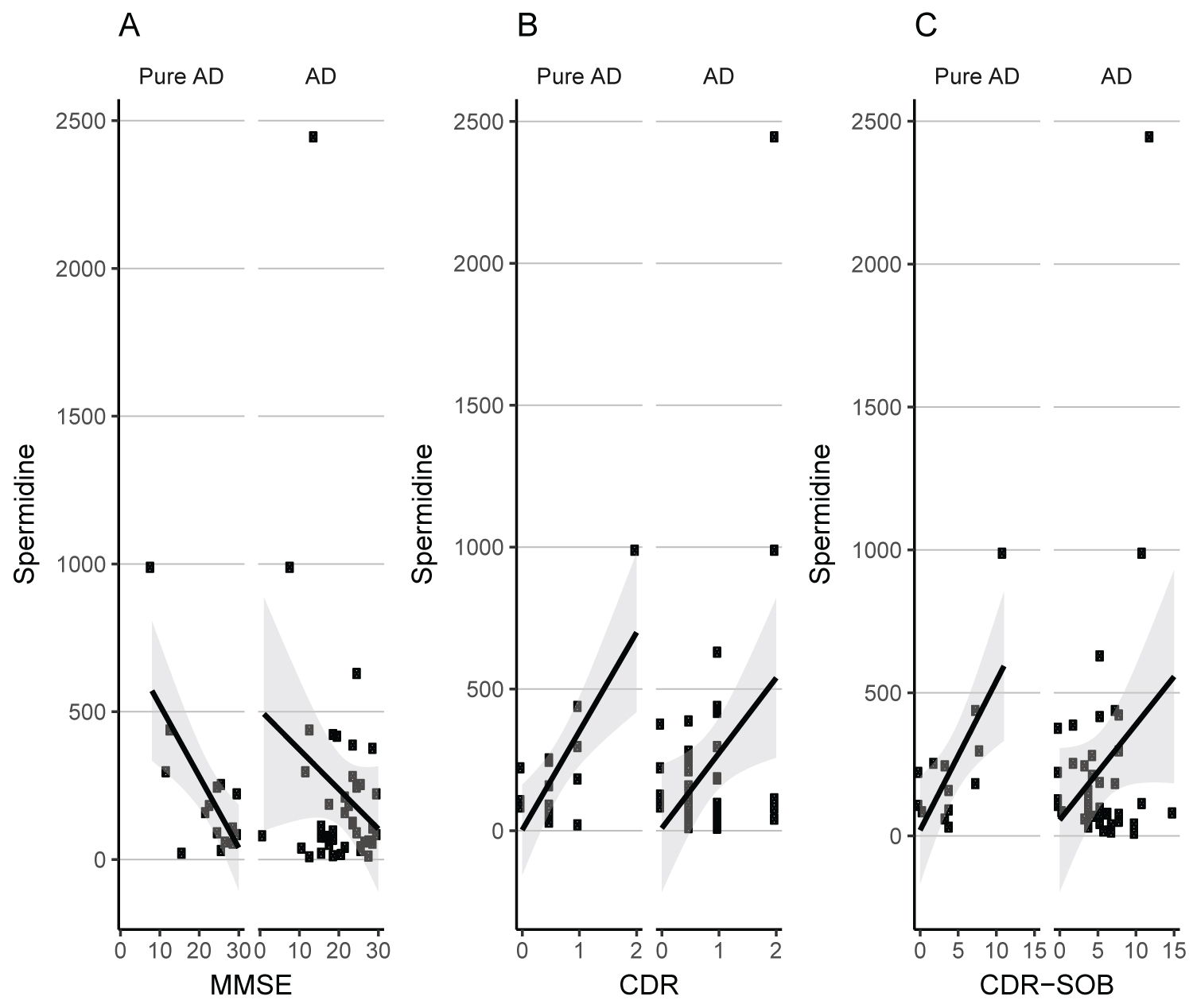
In the pure AD subgroup, a strong correlation was observed between Spermidine serum levels and MMSE (r = -0.705, P = 0.003), CDR (r = 0.751, P = 0.002), and CDR-SOB (r = 0.704, P = 0.007). This correlation was weaker when examined for the total AD group (MMSE: r = -0.21, P = 0.16; CDR: r = 0.36, P = 0.02; CDR-SOB: r = 0.28, P = 0.08) (Figure 2). We did not observe a significant correlation between Spermidine and APOE4, or neuroimaging variables (Table 3). In addition, this correlation was not significant in MCI and controls (results not shown).
The correlation between Spermidine serum levels and iron-related proteins in a subgroup of 24 participants showed no significant association between spermidine and hepcidin (r = 0.31, P = 0.15). Due to the importance of hepcidin as a chief iron regulatory protein, we further analyzed the correlation between Spermidine and hepcidin in a subgroup with advanced disease. Stratification based on cognitive assessment tests, led to a significant correlation between Spermidine and hepcidin serum levels in participants with MMSE of 8-19 (n = 14) (r = 0.604, P = 0.02) (ranging from 8-26), and CDR-SOB of 6-12 (n = 12) (r = 0.601, P = 0.03) (ranging from 3.5-12) (Table 3). We could not perform the same analysis for CDR, since only 4 patients had CDR of 2or higher. Spermidine serum levels did not correlate with the serum levels of other iron-related proteins and with the serum levels of Aβ (Table 3).
Discussion
This pilot study compared serum levels of Spermidine between AD, MCI, and controls. We report significantly higher Spermidine serum levels in MCI group compared to controls. However, the differences in Spermidine serum levels between AD and controls and pure AD and controls did not reach statistical significance. Using mass spectrometry, Graham, et al. reported increased spermidine plasma levels in MCI patients who subsequently converted to AD compared to MCI patient who did not [14]. However, our small sample size did not provide an adequate statistical power for similar analysis. The higher Spermidine serum levels at the dormant stage of the disease, and the correlation between Spermidine and AD indicators of disease severity suggests a possible role of Spermidine in AD pathophysiology. The absence of correlation between Spermidine and Aβ and neuroimaging data may simply be a reflection of the complexity surrounding AD. Furthermore, the correlation between serum polyamines and AD clinical and neuroimaging biomarkers may follow a dynamic pattern that our cross-sectional study is unable to capture in this limited sample size.
We observed higher mean Spermidine serum levels in both controls and AD group compared to the values reported by other investigators for < 65-year-old healthy control subjects with normal body mass index (18‐36 ng/ml) [15]. The discrepancy may have been resulted from differences in the method of measurement, since we used ELISA, whereas mass spectrometry was used in the other study. In addition, both AD and controls in our study were relatively older, had comorbid conditions, and were using psychotropic and cardiovascular drugs with the potential for influencing Spermidine serum levels [16-18].
There was a large inter-individual variability in Spermidine serum levels among AD group, which likely contributed to the observed lack of statistical significance between this group and controls. Calculations showed a requirement for future sample size of approx. n = 65 in each group (AD and control), in order to obtain 90% chance of demonstrating statistically significant group differences in Spermidine serum levels. The reason for the inter-individual variability is unknown, but is unlikely to be related to the freeze-thawing or the long-term storage of the samples, since polyamine levels seem not to be affected by these conditions [19]. A high inter-individual variability in Spermidine serum levels has been also observed in the study of cancer patients [20].
Cellular polyamine homeostasis is maintained through tight regulation of biosynthesis from amino acids, absorption from the intestinal flora and cellular influx and efflux involving multiple feedback mechanisms [21]. The increase in MCI serum levels of Spermidine may be secondary to an increase in the activities of the rate limiting enzymes involved in Spermidine biosynthesis such as SAMDC and ODC, resulting in the increase excretion and a rise in Spermidine serum levels in the circulation [22]. SAMDC and ODC activities have been shown to be upregulated in the AD brain [7,8], but no studies have measured their activities in peripheral tissues. Although in normal physiological condition the transport of polyamines across the blood brain barrier (BBB) is limited [23], the leakage from CNS due to a compromised BBB integrity apparent in MCI stage [24] could increase Spermidine serum levels.
A number of enzymes regulate the secretion of polyamines into the circulation, one of which is Spermidine/spermine-N1 acetyltransferase (SSAT1). This enzyme is able to add acetyl group to the aminopropyl ends of Spermidine and spermine, reducing their binding to macromolecules, and promoting their excretion from the cell into the circulation. SSAT1 has not been investigated in AD or MCI. However, AD dysregulation in iron related proteins such as hepcidin [12] has the potential upregulating SSAT1, increasing Spermidine serum levels [13].
Inflammatory milieu promotes SAMDC activation [25], and inflammatory mediators such as tumor necrosis factor (TNF)-α are known to upregulate SSAT activity [26]. The increase in Spermidine serum levels in MCI patients may be secondary to the inflammation-induced increase in SAMDC and or SSAT activities [27]. However, the notion that NSAIDs, such as aspirin, exert clinical effects, in part, via increasing SSAT activity [17], suggests that the increase in Spermidine in the dormant stage of the disease may be one of the many strategies to reduce inflammation.
This assumption is further supported by in vitro studies showing that Spermidine treatment of microglial cells, the primary cells involved in CNS inflammatory responses, reduces the production of pro-inflammatory mediators in response to lipopolysaccharide (LPS) exposure [28]. Similar anti-inflammatory effects of Spermidine have been shown in LPS-stimulated macrophages [29]. Three months Spermidine supplementation, in the form of Spermidine-rich plant extracts, has been shown to enhance memory performance in adults at risk of dementia [30]. Therefore, the increase in Spermidine may serve as a compensatory physiological response to minimize cognitive decline.
We observed a correlation between spermidine and the chief iron regulatory protein, hepcidin in the AD subgroup with more advanced disease, indicated by cognitive assessment tests, MMSE, and CDR-SOB. These results corroborate the link between polyamines' biosynthesis and metabolism, and iron [13], and suggest a possible dysregulation of both polyamines and hepcidin in the AD pathophysiology. However, these results are by no means conclusive due to the small sample size.
Study limitations
Our results suggest that Spermidine may represent one in the complex pathophysiology of polyamines in AD. However, one should note the study limitations, one of which is the small sample size, especially when participants are stratified to subgroups. In addition we do not have the data for serum levels of spermine in AD, and whether it follows patterns similar to Spermidine. The increase in Spermidine in the MCI group, may be secondary to reduction in the activity of the enzyme spermine synthase which converts Spermidine to spermine, as described in the Introduction. Additional studies with larger sample size are required for better understanding of the role of polyamines in AD pathophysiology, and its utility as a biomarker in preclinical and clinical stages of AD.
Acknowledgment
This study was supported in part by a grant from NIH (P30 AG008017) and grants from The Jog For The Jake Foundation, Buffalo, NY, University of Buffalo. The authors thank Ms. Robin Guariglia, and Ms. Babett Lind, from the Layton Aging and Alzheimer's Research Center, Portland, Oregon, for their assistance and provision of samples.
Authors' Declaration
Authors have no conflict of interest to declare.
References
- Alzheimer's Association (2016) 2016 alzheimer's disease facts and figures. Alzheimers Dement 12: 459-509.
- McKhann GM, Knopman DS, Chertkow H, et al. (2011) The diagnosis of dementia due to alzheimer's disease: Recommendations from the national institute on aging-alzheimer's association workgroups on diagnostic guidelines for alzheimer's disease. Alzheimers Dement 7: 263-269.
- Petersen RC (2011) Clinical practice. Mild cognitive impairment. N Engl J Med 364: 2227-2234.
- Bae DH, Lane DJR, Jansson PJ, et al. (2018) The old and new biochemistry of polyamines. Biochim Biophys Acta Gen Subj 1862: 2053-2068.
- Lenis YY, Elmetwally MA, Maldonado-Estrada JG, et al. (2017) Physiological importance of polyamines. Zygote 25: 244-255.
- Inoue K, Tsutsui H, Akatsu H, et al. (2013) Metabolic profiling of alzheimer's disease brains. Sci Rep 3: 2364.
- Morrison LD, Bergeron C, Kish SJ (1993) Brain s-adenosylmethionine decarboxylase activity is increased in alzheimer's disease. Neurosci Lett 154: 141-144.
- Morrison LD, Cao XC, Kish SJ (1998) Ornithine decarboxylase in human brain: Influence of aging, regional distribution, and alzheimer's disease. J Neurochem 71: 288-294.
- Luo J, Yu CH, Yu H, et al. (2013) Cellular polyamines promote amyloid-beta (abeta) peptide fibrillation and modulate the aggregation pathways. ACS Chem Neurosci 4: 454-462.
- Shankar GM, Li S, Mehta TH, et al. (2008) Amyloid-beta protein dimers isolated directly from alzheimer's brains impair synaptic plasticity and memory. Nat Med 14: 837-842.
- Gonzalez-Dominguez R, Garcia A, Garcia-Barrera T, et al. (2014) Metabolomic profiling of serum in the progression of alzheimer's disease by capillary electrophoresis-mass spectrometry. Electrophoresis 35: 3321-3330.
- Sternberg Z, Hu Z, Sternberg D, et al. (2017) Serum hepcidin levels, iron dyshomeostasis and cognitive loss in alzheimer's disease. Aging Dis 8: 215-227.
- Lane DJR, Bae DH, Siafakas AR, et al. (2018) Coupling of the polyamine and iron metabolism pathways in the regulation of proliferation: Mechanistic links to alterations in key polyamine biosynthetic and catabolic enzymes. Biochim Biophys Acta Mol Basis Dis 1864: 2793-2813.
- Graham SF, Chevallier OP, Elliott CT, et al. (2015) Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and l-arginine metabolism in mild cognitive impairment subjects converting to alzheimer's disease. PLoS One 10: e0119452.
- Song J, Shan Z, Mao J, et al. (2019) Serum polyamine metabolic profile in autoimmune thyroid disease patients. Clin Endocrinol 90: 727-736.
- Turecki LMFaG (2008) Implication of the polyamine system in mental disorders. J Psychiatry Neurosci 33: 102-110.
- Babbar N, Gerner EW, Casero RA, et al. (2006) Induction of spermidine/spermine n1-acetyltransferase (ssat) by aspirin in caco-2 colon cancer cells. Biochem J 394: 317-324.
- Podhorecka M, Ibanez B, Dmoszynska A (2017) Metformin - its potential anti-cancer and anti-aging effects. Postepy Hig Med Dosw (Online) 71: 170-175.
- Magnes C, Fauland A, Gander E, et al. (2014) Polyamines in biological samples: Rapid and robust quantification by solid-phase extraction online-coupled to liquid chromatography-tandem mass spectrometry. J Chromatogr A 1331: 44-51.
- Byun JA, Choi MH, Moon MH, et al. (2009) Serum polyamines in pre- and post-operative patients with breast cancer corrected by menopausal status. Cancer Lett 273: 300-304.
- Park MH, Igarashi K (2013) Polyamines and their metabolites as diagnostic markers of human diseases. Biomol Ther (Seoul) 21: 1-9.
- Bachrach U (2005) Naturally occurring polyamines: Interaction with macromolecules. Curr Protein Pept Sci 6: 559-566.
- Shin WW, Fong WF, Pang SF, et al. (1985) Limited blood-brain barrier transport of polyamines. J Neurochem 44: 1056-1059.
- Ott BR, Jones RN, Daiello LA, et al. (2018) Blood-cerebrospinal fluid barrier gradients in mild cognitive impairment and alzheimer's disease: Relationship to inflammatory cytokines and chemokines. Front Aging Neurosci 10: 245.
- Noguchi Y, Meyer T, Tiao G, et al. (1996) Sepsis increases putrescine concentration and protein synthesis in mucosa of small intestine in rats. Shock 5: 333-340.
- Babbar N, Hacker A, Huang Y, et al. (2006) Tumor necrosis factor alpha induces spermidine/spermine n1-acetyltransferase through nuclear factor kappab in non-small cell lung cancer cells. J Biol Chem 281: 24182-24192.
- Magalhaes TNC, Weiler M, Teixeira CVL, et al. (2018) Systemic inflammation and multimodal biomarkers in amnestic mild cognitive impairment and alzheimer's disease. Mol Neurobiol 55: 5689-5697.
- Choi YH, Park HY (2012) Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated bv2 microglial cells. J Biomed Sci 19: 31.
- Van den Bossche J, Lamers WH, Koehler ES, et al. (2012) Pivotal advance: Arginase-1-independent polyamine production stimulates the expression of il-4-induced alternatively activated macrophage markers while inhibiting lps-induced expression of inflammatory genes. J Leukoc Biol 91: 685-699.
- Wirth M, Benson G, Schwarz C, et al. (2018) The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex 109: 181-188.
Corresponding Author
Zohi Sternberg, PhD, Clinical Associate Professor of Neurology and Neurosurgery, Department of Neurology, Stroke Center, Buffalo Medical Center, Buffalo, NY, 14203, USA, Tel: 716-8597540, Fax: 716-8592430; 859-7573.
Copyright
© 2021 Sternberg Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.